Water
Water has structural and chemical properties which make it highly useful to living cells. Water is an abundant and important inorganic compound, vital to living organisms. All living organisms require it, among a wide variety of other inorganic compounds for growth, repair, maintenance, and reproduction. No organism can survive without water. Water can make up anywhere from 5-95% of cells, averaging 65-75% of most cells. Water facilitates penetration of nutrients through cell membranes and inside the cell it is the medium for most chemical reactions.
Water's Polarity
Water is a polar molecule, it has a negative and positive charge.
Water, or H20, has an unequal distribution of charges making it easy for its molecules to form bonds. It is an excellent temperature shield due to its hydrogen bond between water molecules.

Hydrogen bonding between water molecules makes water an excellent temperature shield for organisms.

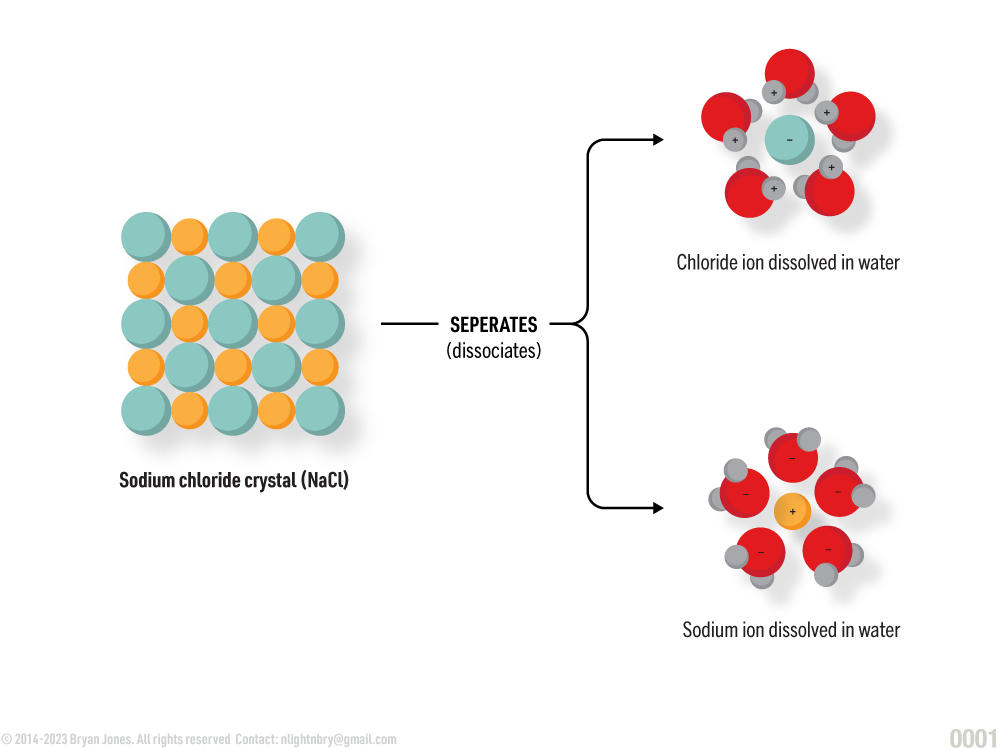
Many polar molecules separate in water making water an excellent dissolving medium or solvent.

Water Ionizating Sodium Chloride
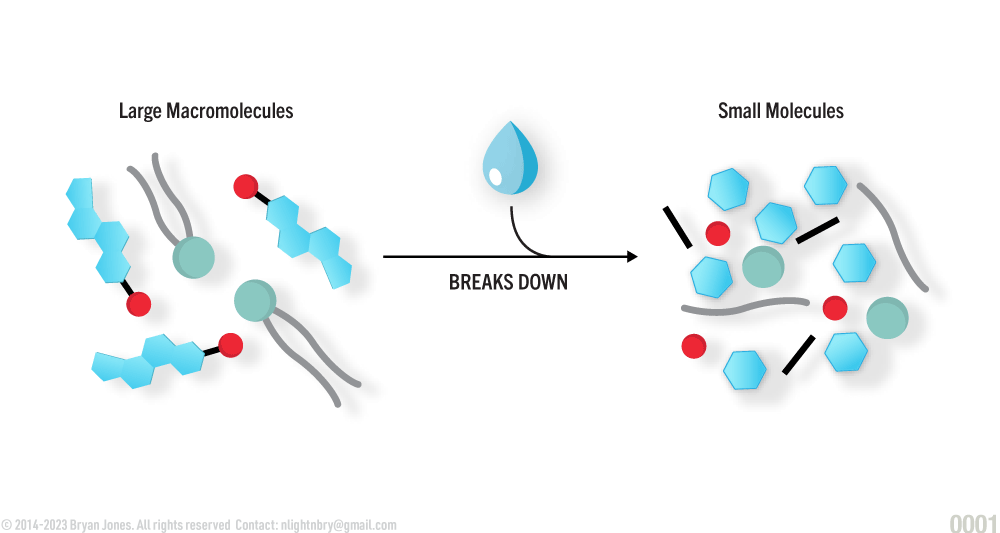
Water as a Reactant
Water is required for catabolism
Water is necessary in the breaking down of larger molecules into smaller ones. The reaction is called Hydrolysis.
Catabolism
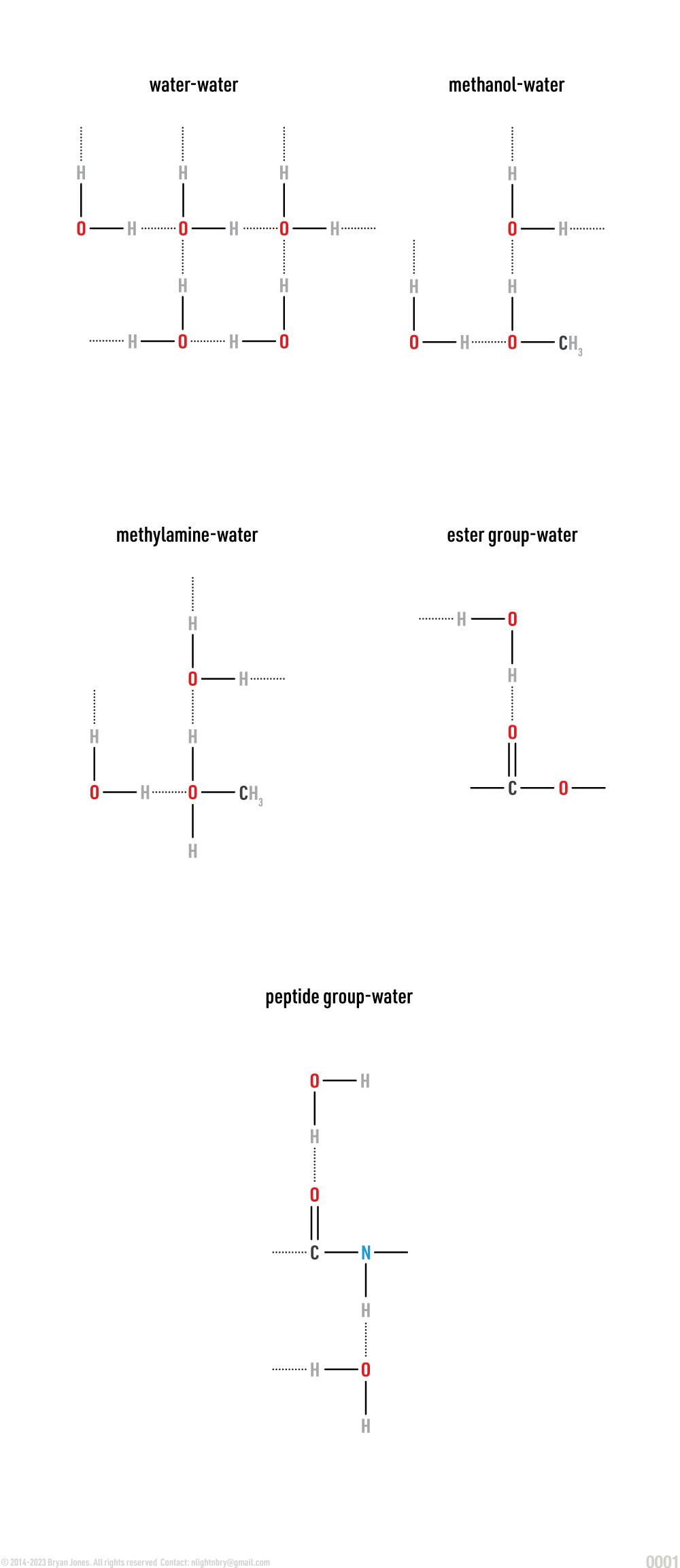
Water Networks
Water molecules arrange differently based on many factors such as pH, elemental composition, and pressure.
Network Structure

Types of Network Structure Arrangements