Elements of Life
Living organisms require certain elements in combinations known as organic compounds. All organic compounds contain carbon. Here are some of the common elements of life:
Elements of Life
Hydrogen
Hydrogen is another important element of life, often found in combination with carbon. Hydrocarbons are compounds containing only carbon and hydrogen.
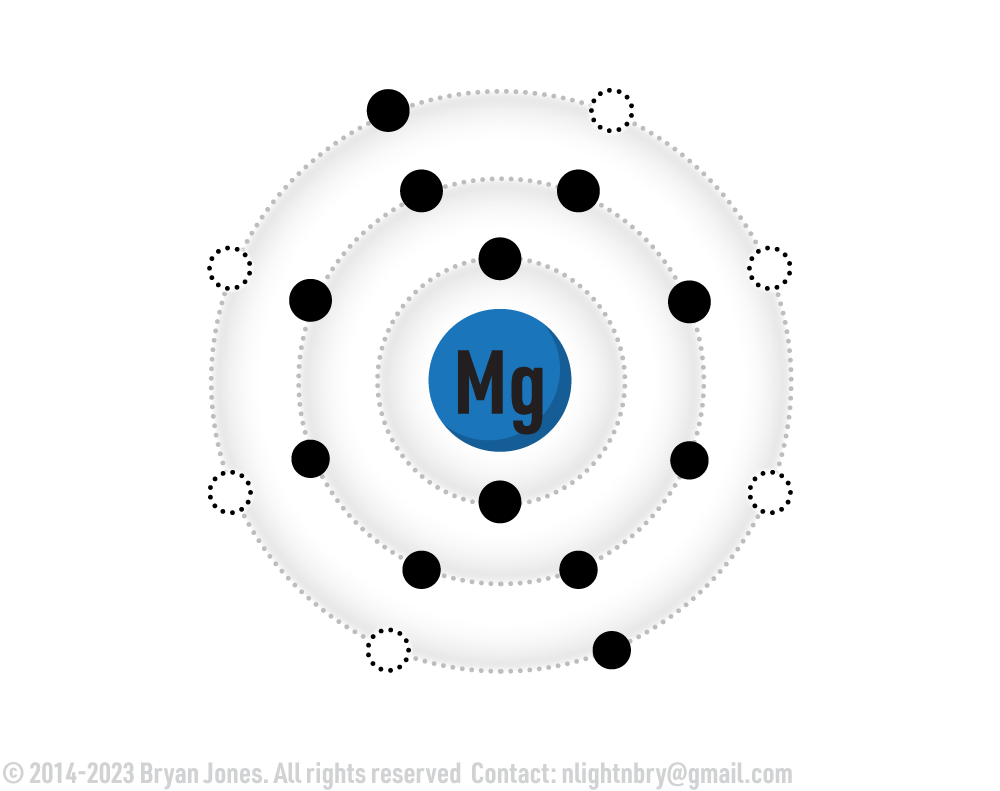
Hydrogen is an important element in all organic (and some inorganic) compounds. These include water, salts, and certain naturally occurring gases. Microbes both use and produce these gases. Hydrogen has several overlapping roles in cells:
- Maintains pH.
- Forms hydrogen bonds between molecules.
- Serves as energy for oxidation reduction reactions.
Hydrogen:
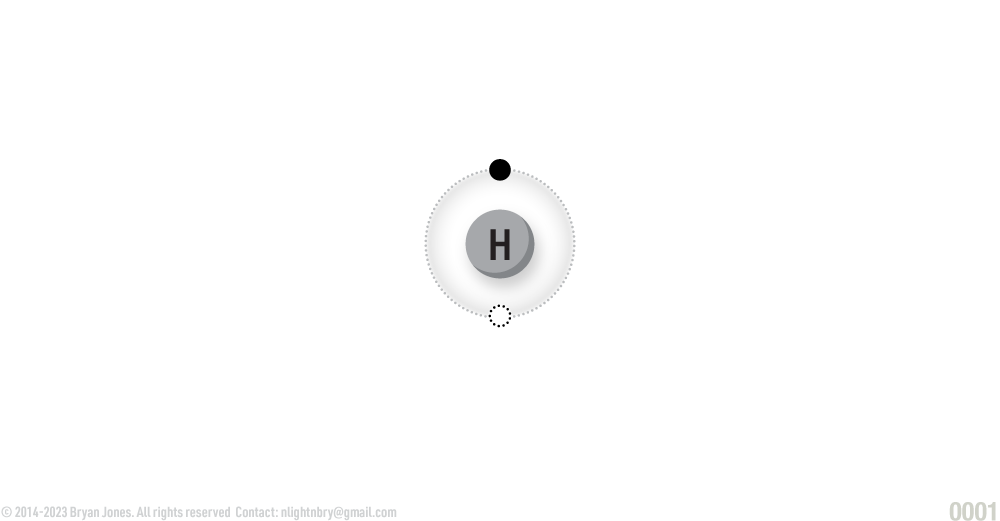
ATOMIC NUMBER: 1
NUMBER OF ELECTRONS IN EACH ENERGY LEVEL: 1
ATOMIC MASS: 1.00
Carbon
Carbon bonds are basic structures for building life.
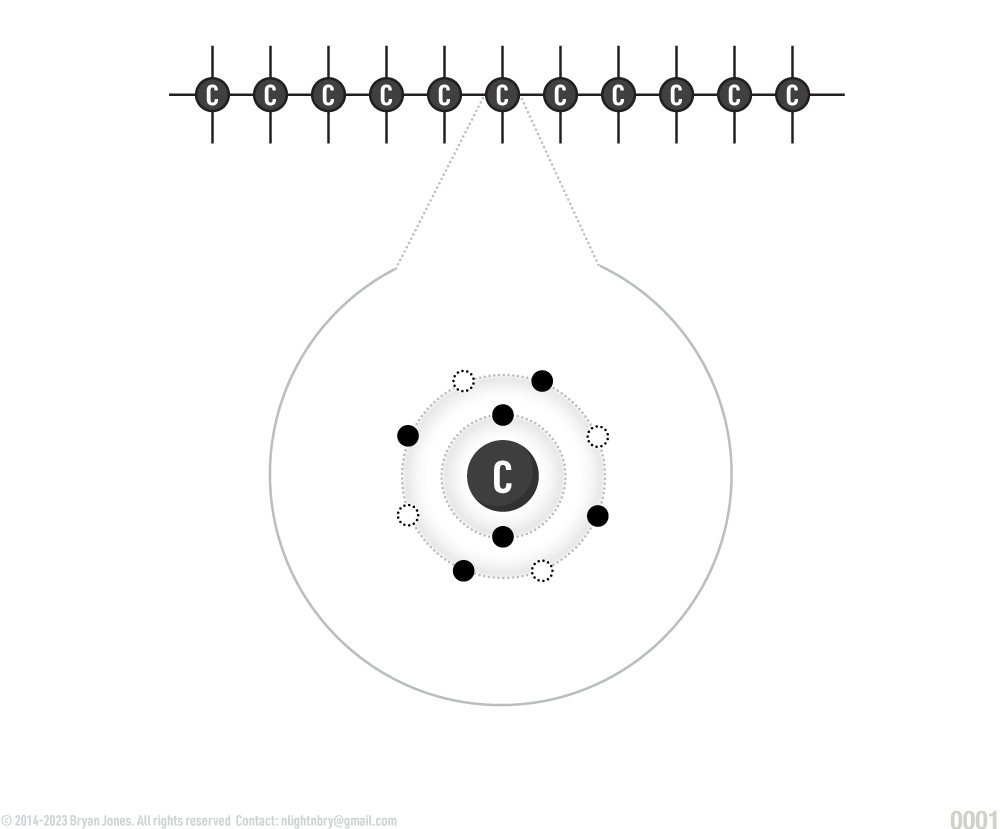
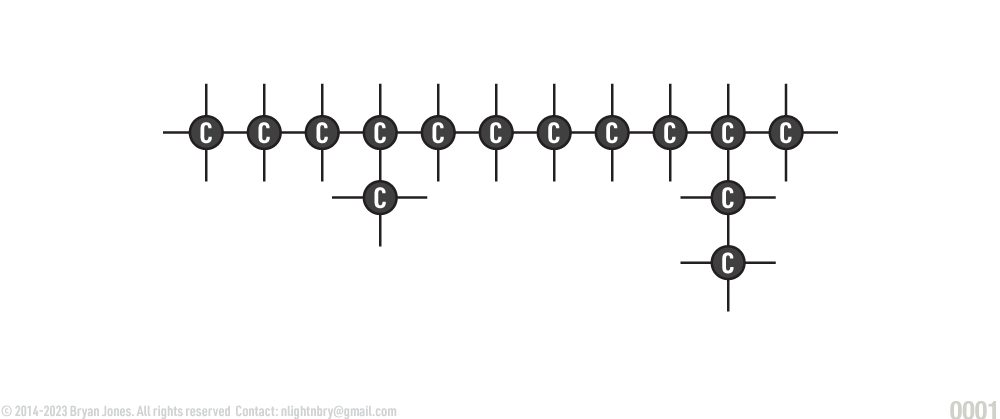
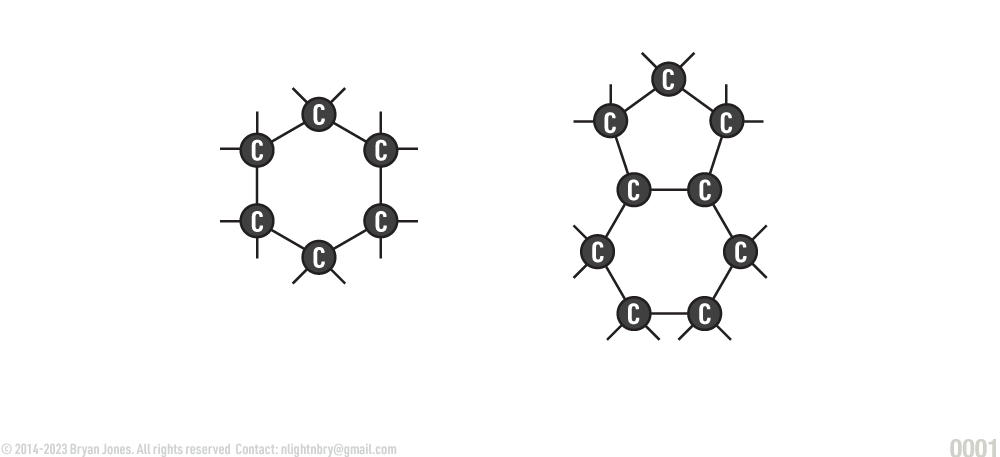
Carbon forms the backbone of organic molecules. Carbon has four electrons in its outer ring (called an orbital). This makes it ideal for covalent bonds with other atoms. This ability to covalently bond allows carbon to form stable chains containing thousands of carbon atoms and still has sites available for forming bonds with other atoms. A single carbon atom can form four single bonds, two double bonds, or one triple bond. The covalent bonds that carbon forms are linear, branched, or ringed. The chain of carbon atoms in organic molecules is called a carbon skeleton which is the backbone of organic molecules.
Carbon:
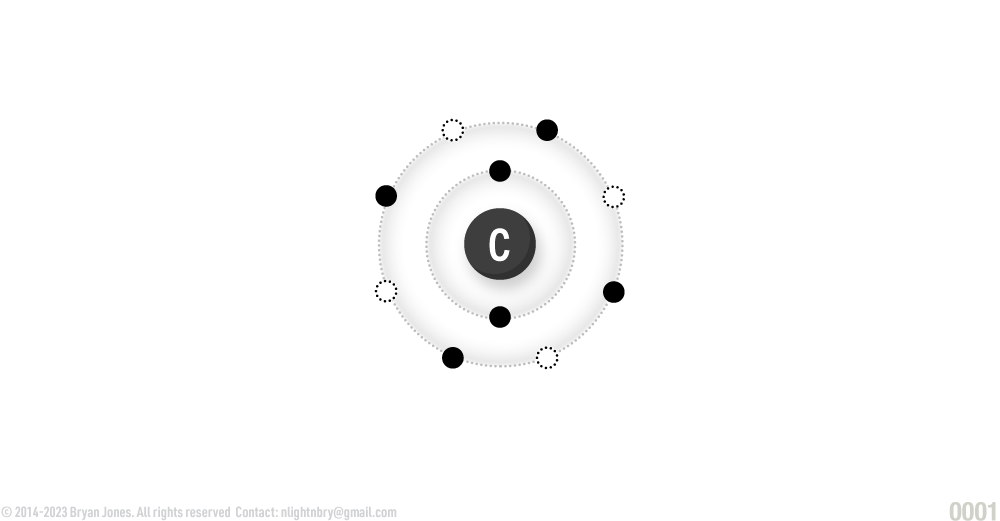
ATOMIC NUMBER: 8
NUMBER OF ELECTRONS IN EACH ENERGY LEVEL: 2 • 6
ATOMIC MASS: 14.0
Carbon Structures:
Nitrogen
The main source of nitrogen on Earth is nitrogen gas (N2) located in the atmosphere, other sources include underground reservoirs often a byproduct of decaying organic matter. This element is indispensable to the structure of proteins, DNA, RNA, and ATP. Bacteria and algae can use inorganic nitrogen compounds for nutrients.
- Nitrogen is an essential component of all proteins. Proteins provide cells with nourishment and for repairing or building new tissue.
- Nitric oxide is composed of nitrogen and oxygen (N2O2). Nitric oxide is used when cells communicate with each other.
- Nitrogen is a key component of urea. Urea is used to remove damaged and used proteins from the body; it removes toxins.
- Nitrogen is also a key component of enzymes. Among other things, it accelerates food metabolism.
Regardless of the initial form in which an inorganic nitrogen enters a cell, it must first be converted to NH3, so that it can directly combine with carbon.
Nitrogen
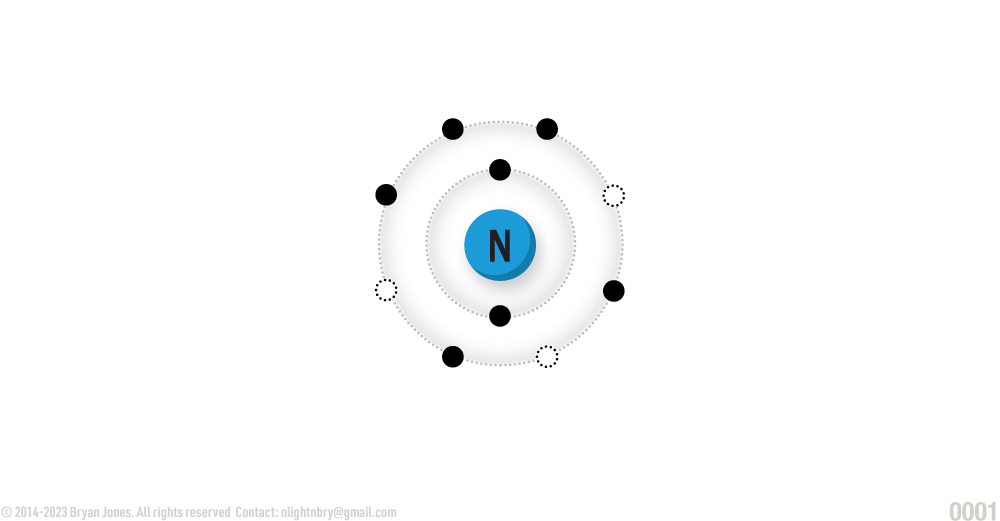
ATOMIC NUMBER: 7
NUMBER OF ELECTRONS IN EACH ENERGY LEVEL: 2 • 5
ATOMIC MASS: 14.0
Oxygen
Oxygen has an important role in the structural and enzymatic functions of the cell. It is a major component of organic compounds such as carbohydrates, lipids, nucleic acids, and proteins. Oxygen is also a common component of water and inorganic salts such as sulfates, phosphates, and nitrates. Free gaseous oxygen (O2) is necessary for metabolism in many organisms.
Oxygen
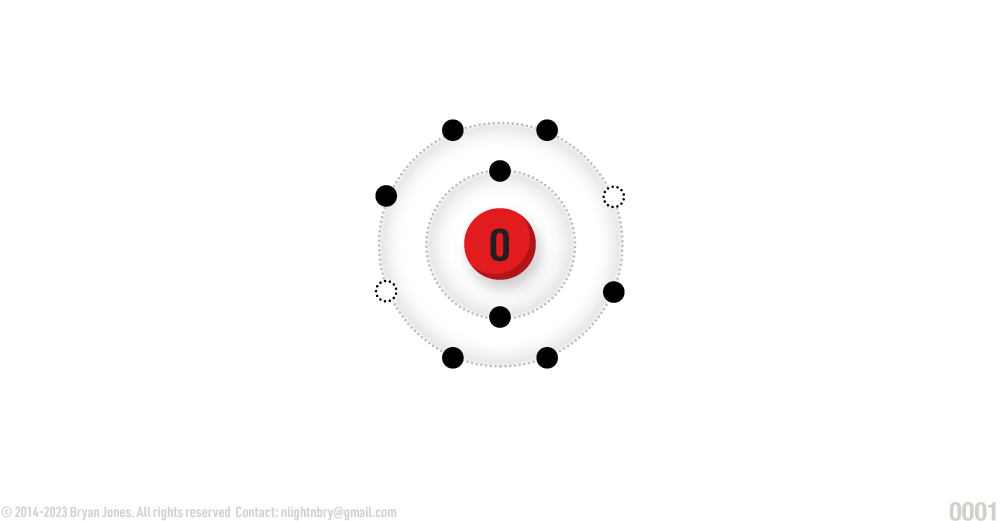
ATOMIC NUMBER: 8
NUMBER OF ELECTRONS IN EACH ENERGY LEVEL: 2 • 6
ATOMIC MASS: 14.0
Phosphorous
The primary inorganic source of phosphorus is phosphate, taken from phosphoric acid (H3PO4). It is common in rocks and ocean mineral deposits. Phosphates are key components of nucleic acids. Because of this, they are essential to cellular genetics and viruses.
Adenosine triphosphate (ATP) is integral to cellular energy transfers. Cell membranes are made of up phosphate-containing compounds called phospholipids. Because phosphates are important to energy transfer as well as genetics, a limitation in availability results in a limitation of growth.
Phosphorous
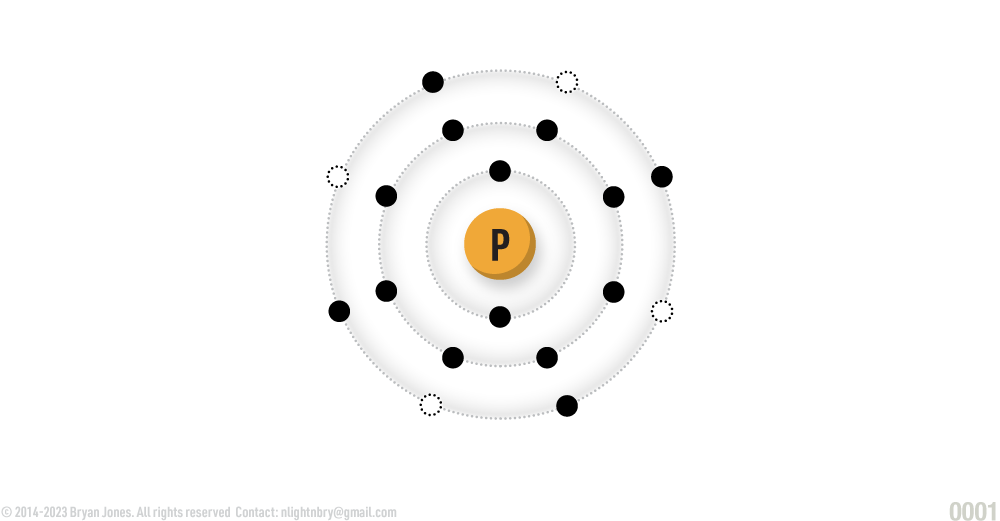
ATOMIC NUMBER: 15
NUMBER OF ELECTRONS IN EACH ENERGY LEVEL: 2 • 8 • 5
ATOMIC MASS: 30.97
Sulfur
Sulfur is critical to humans, plants and bacteria.
Sulfur is commonly distributed in mineral forms. Sulfur has an essential role in some vitamins (such as B1) and many amino acids. Disulfide bonds help determine the shape and structural stability of proteins by their unique linkage.
- An amino acid which contains sulfur is methionine; it is present in all proteins.
- Thiamine (vitamin B1), which contains sulfur, is essential for the peripheral nervous system.
- Sulfur is essential for plant growth, root formation and defense mechanisms.
Sulfur
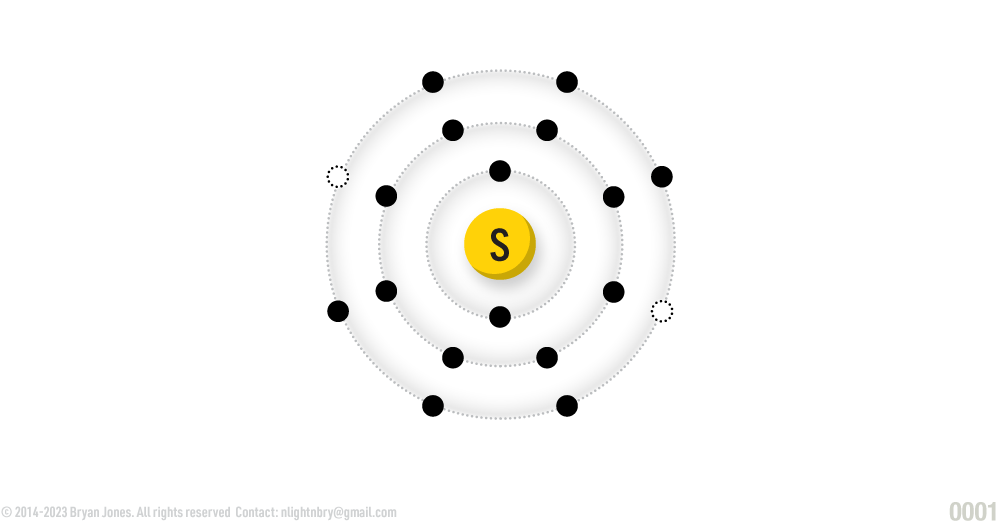
ATOMIC NUMBER: 16
NUMBER OF ELECTRONS IN EACH ENERGY LEVEL: 2 • 8 • 6
ATOMIC MASS: 32.06
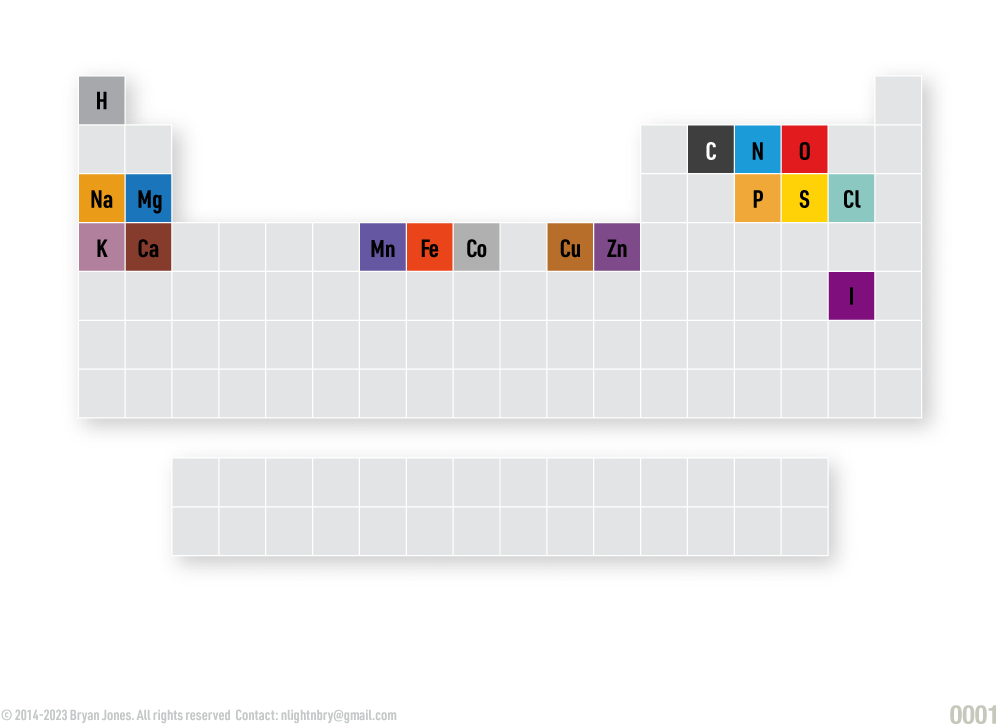
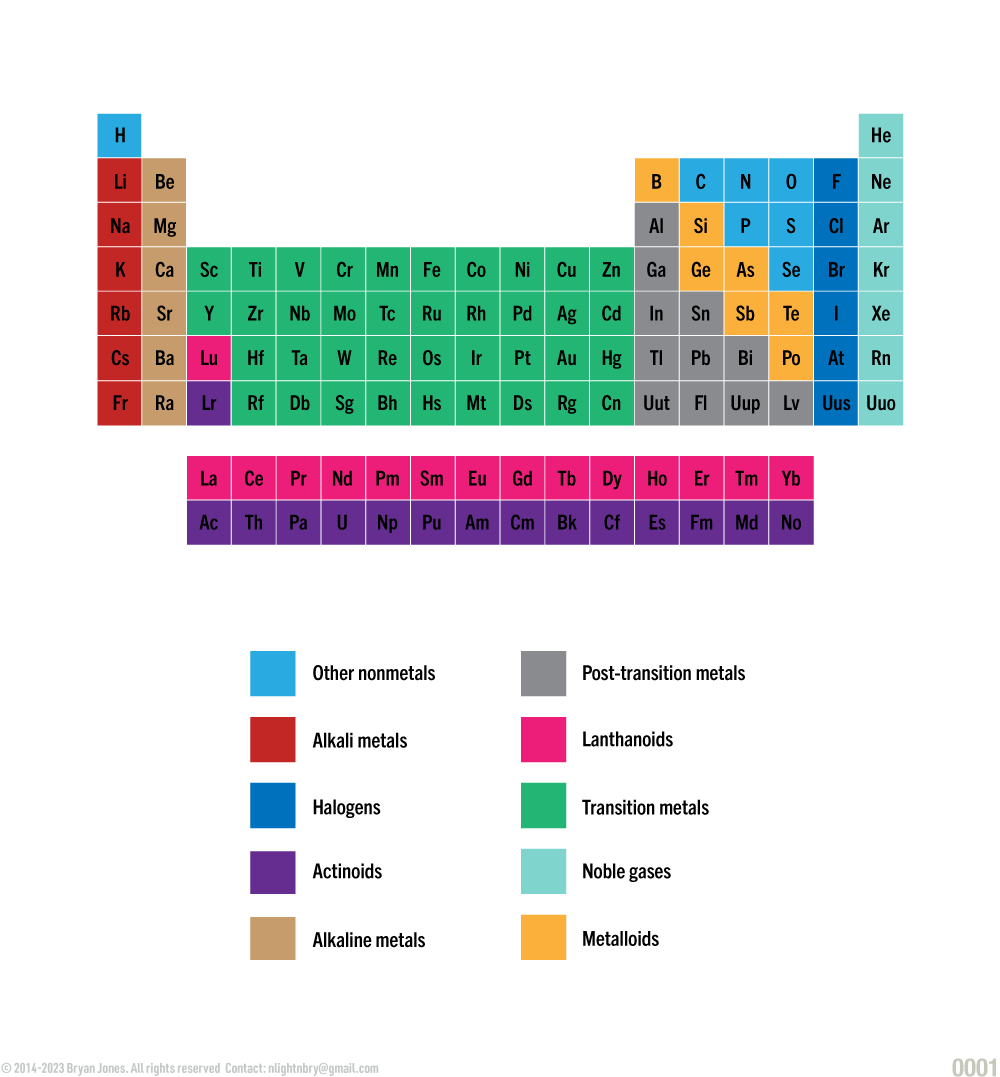
Periodic Table of Elemental Groups
Elements of life are grouped into various categories, the most common being Nonmetals. Others, include Transition metals (electron providers), Alkali and Alkaline metals and Halogens.
If you're wondering if these elements are the only ones that can produce life as we know it, you should research astrobiology. Other elements have been theorized to support life.
Elemental Groups
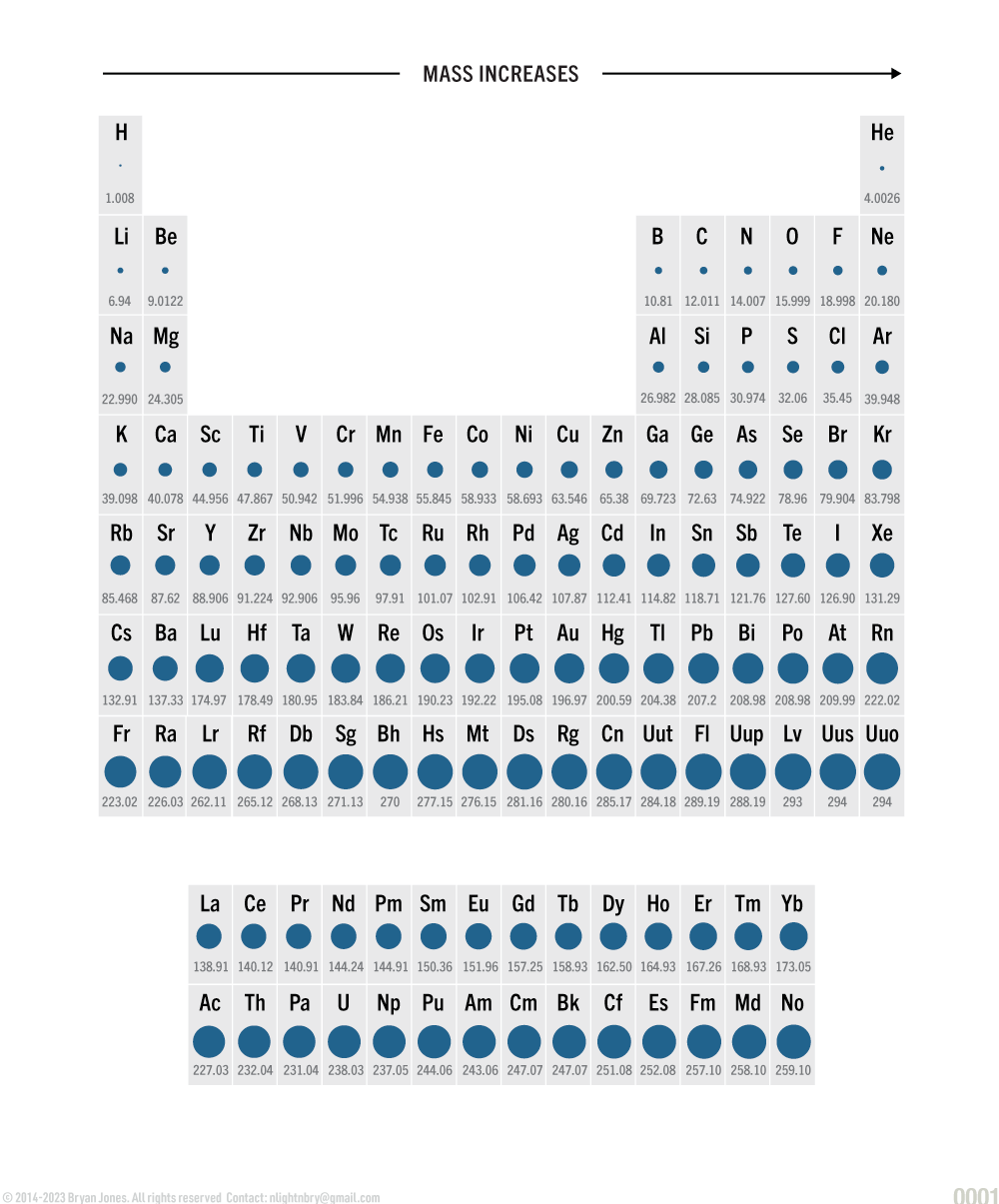
Periodic Table of Atomic Mass
Atomic mass plays a role in life as you might expect, you should notice that most elements of life are between 1.008u and 126.90u. Unified Atomic Mass (u)
Elemental Groups