Lipids
Lipids are a type of biological molecule that are important for a wide range of functions in our bodies. They are often referred to as fats and oils and play important roles in energy storage, cell membrane structure, and signaling.
Lipids are Diverse
Lipids are fats and essential to the structure of a cell.
Lipids are made up of three main components: fatty acids, glycerol, and a variety of other molecules. Fatty acids are long chains of carbon atoms with a carboxyl group at one end. They can be saturated or unsaturated depending on whether all of the carbon atoms in the chain are bonded to hydrogen atoms or not. Glycerol is a small molecule that forms the backbone of many lipids, including triglycerides, which are the main form of fat stored in our bodies. There are several different types of lipids, each with unique structures and functions. Some of the most common types of lipids include:
- Triglycerides: these are the most common type of lipid in our bodies and are used for energy storage.
- Phospholipids: these are the main components of cell membranes and help to keep the cell structure stable.
- Steroids: these are lipids that have a unique four-ring structure and include molecules such as cholesterol and hormones like testosterone and estrogen.
Lipids

Lipid Functions
Here are some functions for now, lipids can be difficult to understand until you essentially have your brain look at it 150+ times. They're a structure and components are added to it can get vastly different affects. For more detail scroll to Lipid Summary.
Fatty Acids
Fatty acids consist of chains of hydrocarbons.
Fatty acids are comprised of several key features, specifically: hydrocarbon chains, carboxyl groups and glycerols. The hydrocarbon chains are long, but made up of only hydrogen and carbon atoms. The carboxyl group exists on one end of the fatty acid, with one free hydrocarbon binding to glycerol.
Conventionally refered to as fatty acids, although probably better understood as acid polymers, You could think of it asa three layer sandwich it which carbon is getting layered by hydrogens from both sides. This way the next person who thinks fat is a bad word can calm down, seriously send your kids to hood school if your're hurt by the word fat. tread or sink, world has a bias.
Naturally occurring fatty acids have a even numbers of carbon atoms in their hydrocarbon chains (typically 4 to 28 carbon atoms).
Among several functions in the body, fatty acids can store energy, which, when metabolized yield ATP in large quantities. However, it is secondary to sugars as a fuel and will only be used once the those resources have been exhausted.
- Alkanes are hydrocarbons with only single bonds between atoms. Alkanes can be either straight-chain or branched-chain. Straight-chain alkanes are the simplest. The number of carbons in each chain determine the name.
- Alkenes are hydrocarbons with at least one double bond. They are named in the same way as alkanes, but instead of an -ane suffix, an -ene.
- Alkynes are hydrocarbons with at least one triple bond.They are named in the same way as alkanes, but instead of an -ane suffix, an -yne.
Mnemonic

Hydrocarbon Structure:
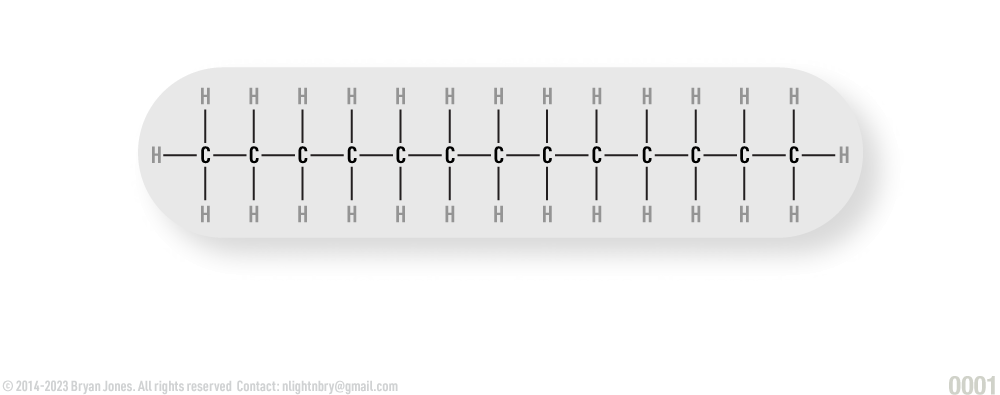
Fatty Acid Structure:

When your bottom lip makes me think of Al kynes of things, like subcutaneous tissue depth, support and unwavering eye-contact. 🪅
Alkanes:
Your lips are precious, and you need to protect them, or you might end of like early french impressionists which is why I'll provide you with some options to prevent inflammation and promote longevity.
Long-chain octylcyanoacrylate-based glues were approved in the U.S. by 1998, but newer versions such as those that utilize acrylate copolymers, poly-vinyl alcohol based hydrogels that could not only shield your skin from potentially toxic pigments found in cosmetics, but also can make your lips fuller, as these compounds asborb water, and depending on the type of polymer ("poly" many "mer" better understood as a unit arranged in a specific way often layers upon each other) can heal your lips.
Silver triethanolamine loaded polymer will ward of vampires and werewolves, It speed of drying, excellent tensile strength and elongation characteristics and water vapor transmission rate are noteable advantages. (1) Ethanal, a polar molecule which interacts with water in the same way magnets orient themselves. Ethanol is bonded to an amine group which is a component of amino acids (proteins) which makes this compound easily integrate into your lipid membranes like three fries pinched together resembling a tripod traveling toward your mouth.
If you're feeling like a carousel horse and find yourself crying, your tears can contribute to fuller lips if you go the hydrogel route.
Some horses will kick you in the face for using technology to change the way in which a carousel spins leading to devasting consequences for those who aided tyranny, of which will likely also need hydrogel bandages, which can absorb up to one-third its weight in blood and fluids over 72 hours. Be sure to fact-check or a horse will kick you again perhaps like a Zebra.
Nano-porous nitrocellulose liquid bandage provides (3) flexibility, softness, transparency, and conformability. May provide a better alternative for lip damage prevention, which is different than it's current application.
New product research should enable printable substrates and protective layers that enable fluorescence and "glow in the dark" paints. Slip-casted liquids that are transparent and those that are white.
References
- Mu, Xiaofeng, et al. "Nano-porous nitrocellulose liquid bandage modulates cell and cytokine response and accelerates cutaneous wound healing in a mouse model."Carbohydrate polymers 136 (2016): 618-629.
- Wax-like Liquid bandage composition https://patents.google.com/patent/GB2414184A/en
- Zhou, F., Wang, W., & Guo, H. (2019). Silver triethanolamine-loaded PVB/CO films for a potential liquid bandage application. Journal of Biomaterials Applications, 33(10), 1434-1443.
Lip's tissue compositions includes
- Epidermis: The outer layer of the lips, which is thinner than that of the surrounding skin. provides a protective barrier against external factors such as microbes, UV radiation, and dehydration
- Dermis: Beneath the epidermis, containing blood vessels, nerves, and connective tissues.
- Submucosa: A layer of loose connective tissue beneath the dermis, providing flexibility to the lips.
Your lips have 3-5 layers or cellular divisions while your "normal" skin has 16.
- Stratum Basale (Basal Layer): The innermost layer where cells actively divide through mitosis. It contains basal cells, melanocytes, and Merkel cells.
- Stratum Spinosum (Prickle Cell Layer): The cells in this layer have a spiky appearance, and they are connected by structures called desmosomes.
- Stratum Granulosum (Granular Layer): Cells in this layer contain granules that have proteins and lipids important for the skin's barrier function.
- Stratum Lucidum (Clear Layer): This layer is not always present, and it is more prominent in areas of thick skin, like the palms and soles. It consists of clear, flat, and densely packed cells.
- Stratum Corneum (Horny Layer): The outermost layer consisting of dead, flattened keratinocytes that form a protective barrier. It helps prevent water loss and protects against external threats.
AVAILABLE SOON: Tissue composition of Lips

Saturated and unsaturated fats:
The difference between saturated and unsaturated fats has to do with the number of bonds between hydrogen atoms and the carbon atoms present in the fatty acid.
When there are no more available places for hydrogens to attach to the molecule, it is known as a saturated fat. When there is still room for more hydrogen atoms, it is unsaturated. The ways in which hydrogen bonds to carbon atoms include single, double and triple bonds. These bonds control the shape of the molecule- unsaturated fats are non-linear, saturated are linear.
Fatty Acid Structure:
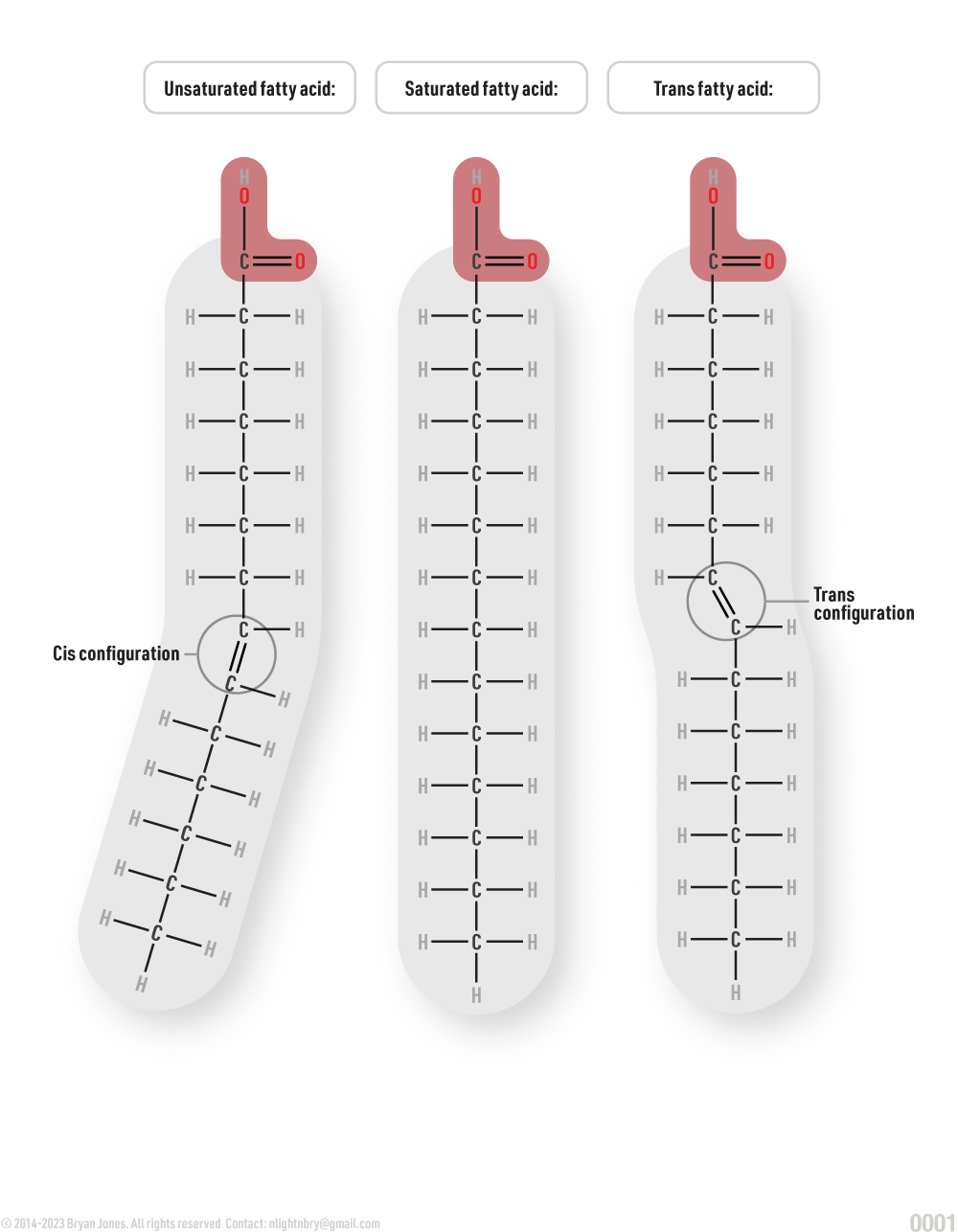
Cis and Trans configuration
Configuration refers to the location of the hydrogen atoms adjacent to the carbon double bond in a molecule. When they are on the same side of the chain, the molecule is called cis (same) and when on opposite sides, it is called trans.
- A double bond is rigid and therefore stabilizes the shape of the molecule.
- Because cis bonds are rigid and cause kinks in the chain of the molecule, the fatty acids may not pack tightly with other molecules.
- Organic compounds cannot switch from cis to trans or the other way around.
Cis and Trans Configurations

Examples of Fatty Acid:
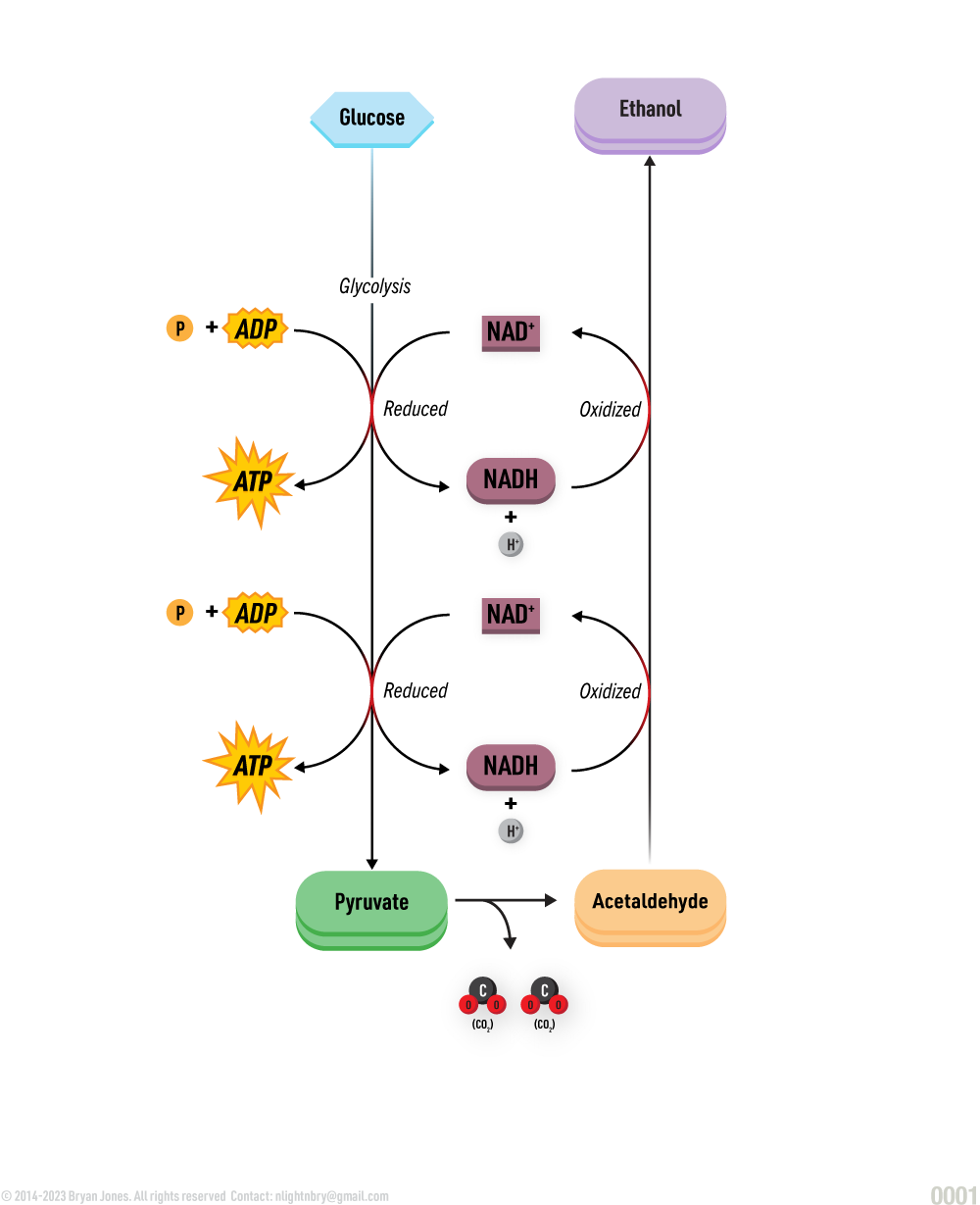
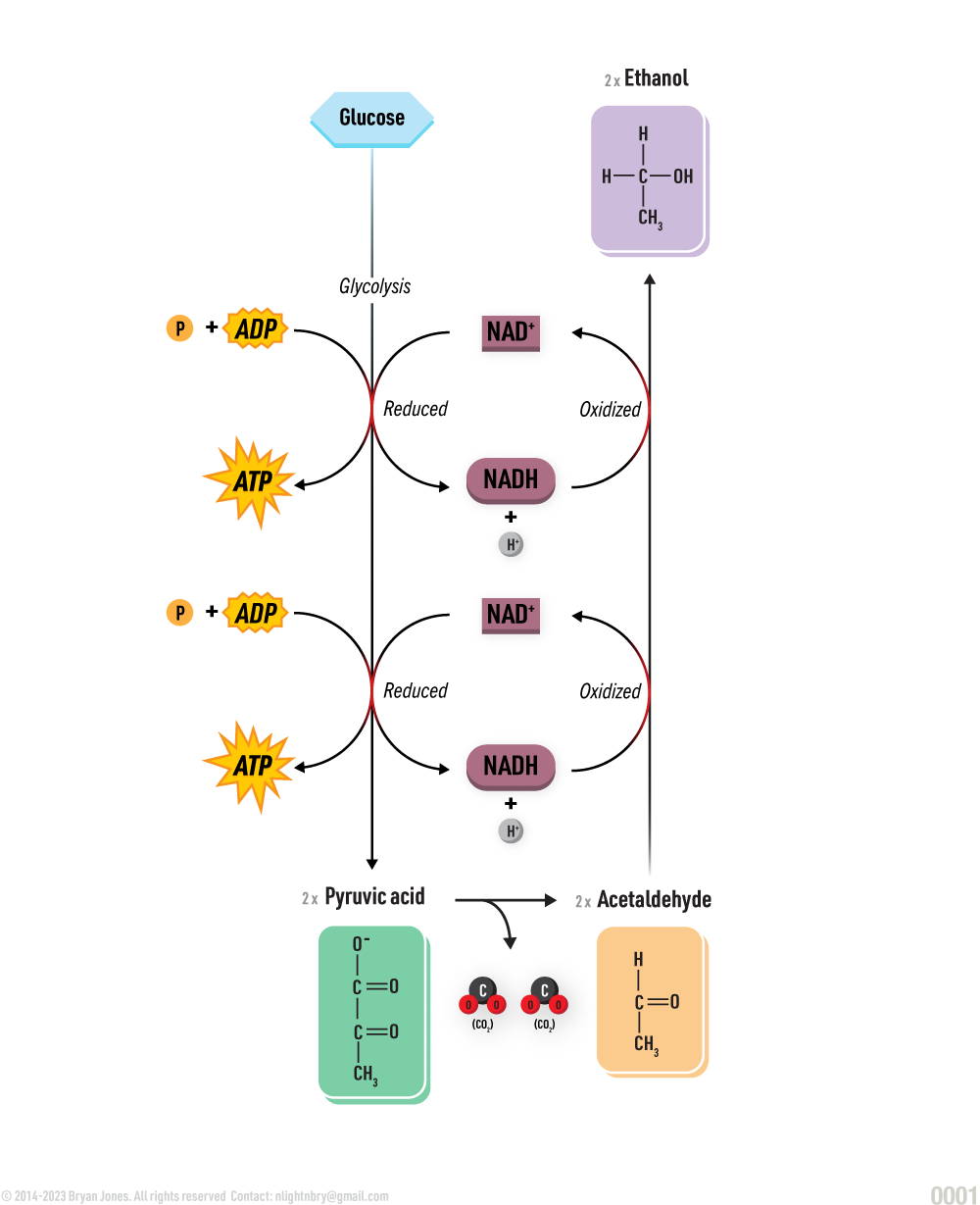
Lipid Synthesis
Glycerol and fatty acids join to form a lipid.
A dehydration reaction is any reaction which results in the loss of a water molecule from the reactants. For example, glycerol combine with a group of compounds known as fatty acids.
Dehydration Synthesis Reaction:
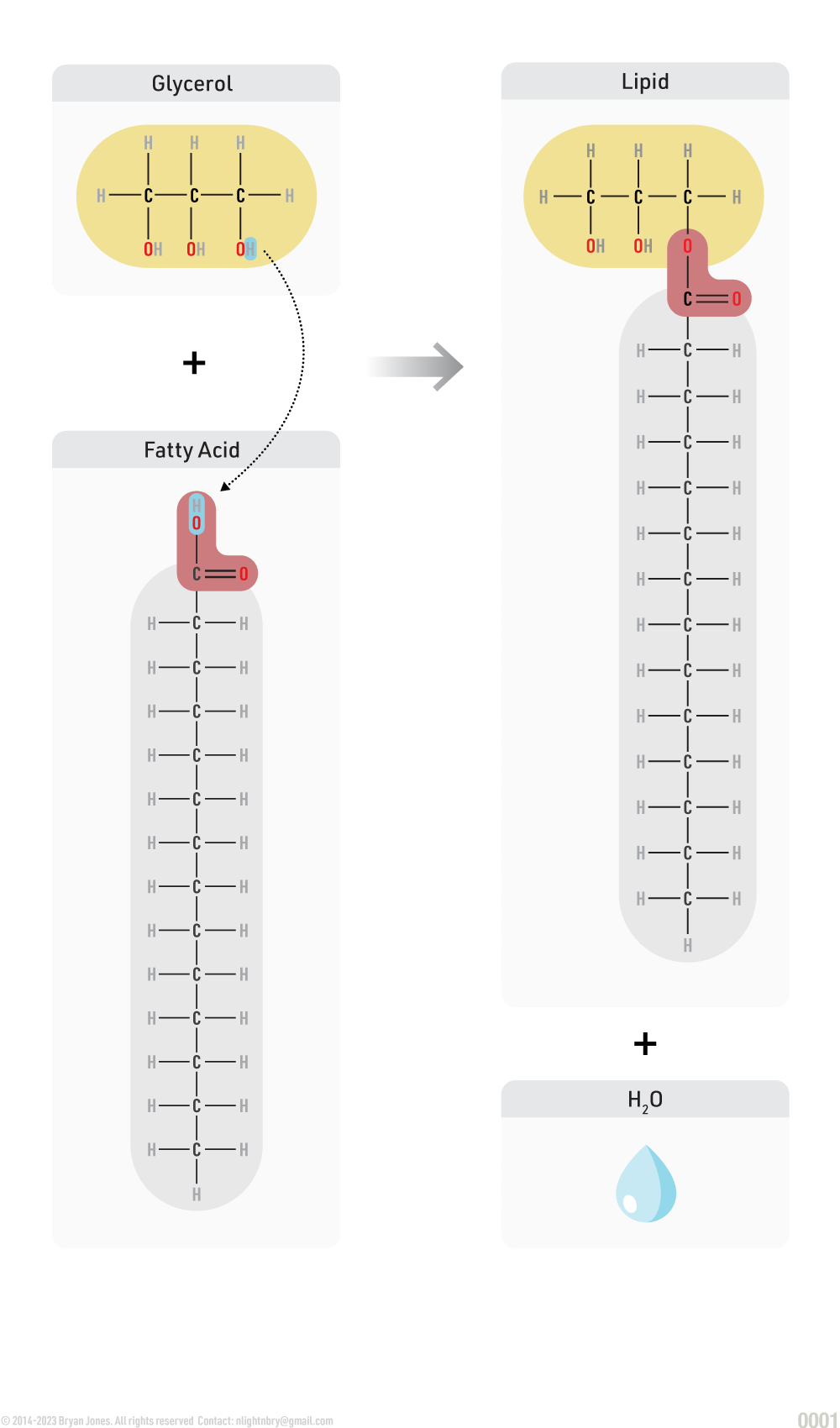
Lipid Anabolism
Synthesis of lipids from simpler molecules
lipid anabolism is the process of building lipids from smaller molecules, such as glucose, amino acids, and glycerol. Lipids are essential for many biological processes, including energy storage, cell signaling, and membrane formation.
Graphic

Simple Lipid
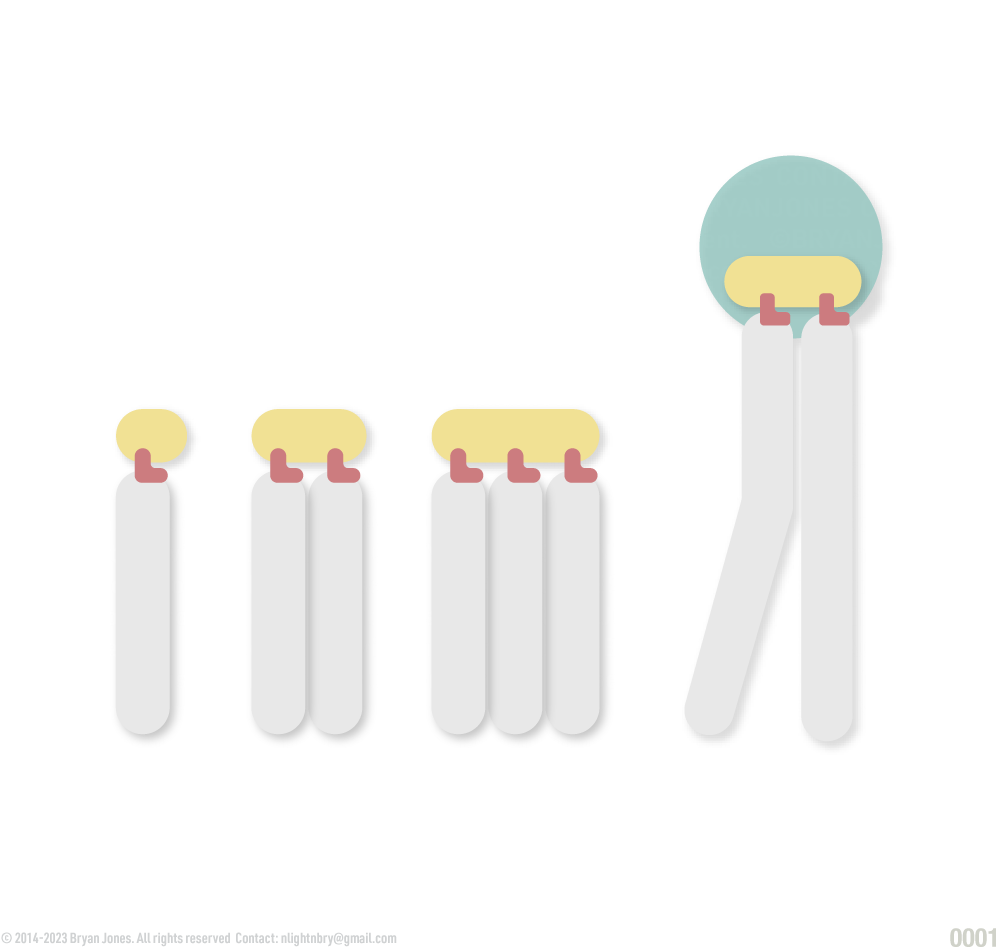
Fats are lipids and consist of monoglycerides, diglycerides, and triglycerides.
Glycerol combines with a group of compounds known as fatty acids. These comprise simple lipids. The number of fatty acid molecules determines whether the fat molecule is a monoglyceride, diglyceride, or triglyceride.
Monoglyceride:
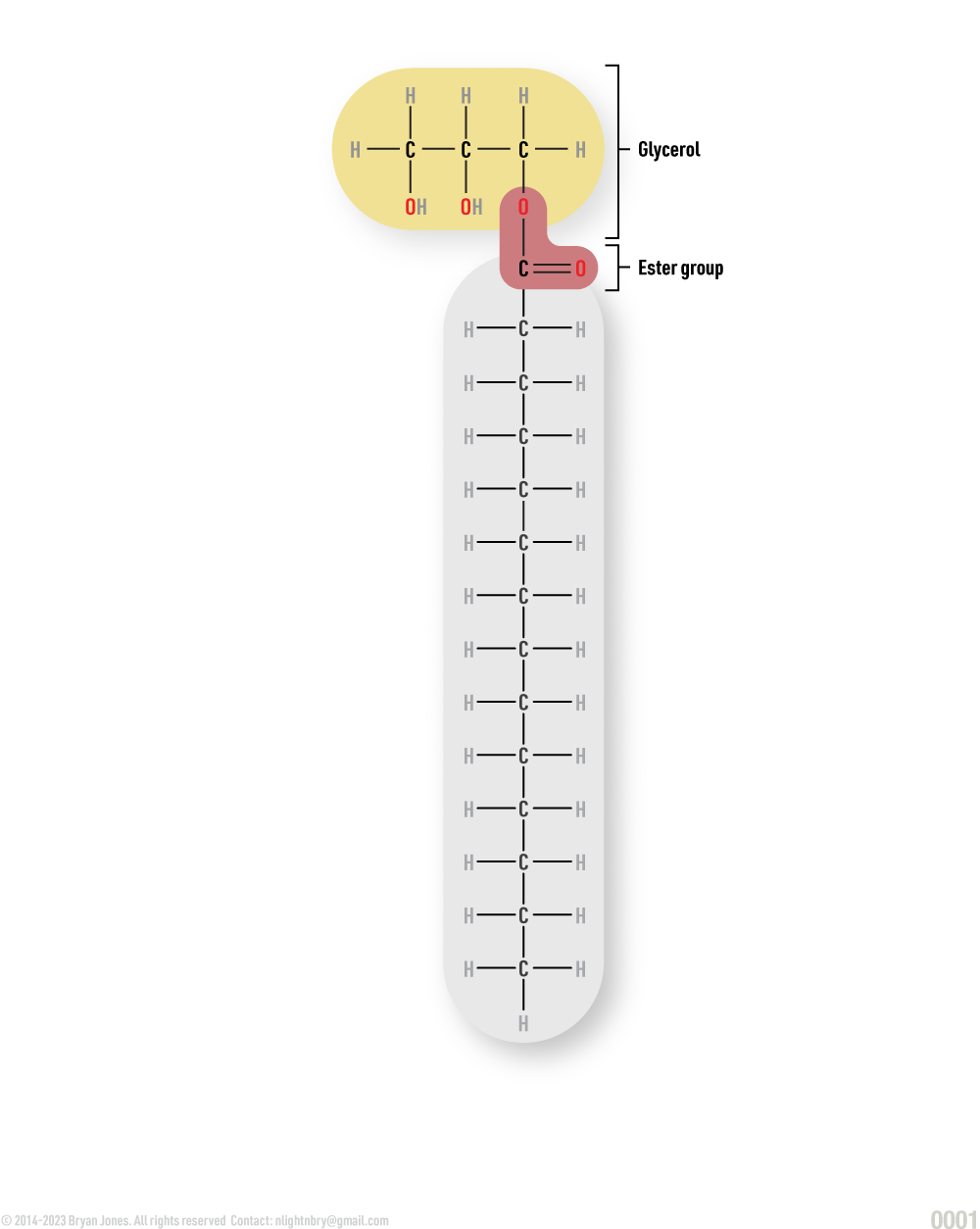
Diglyceride:
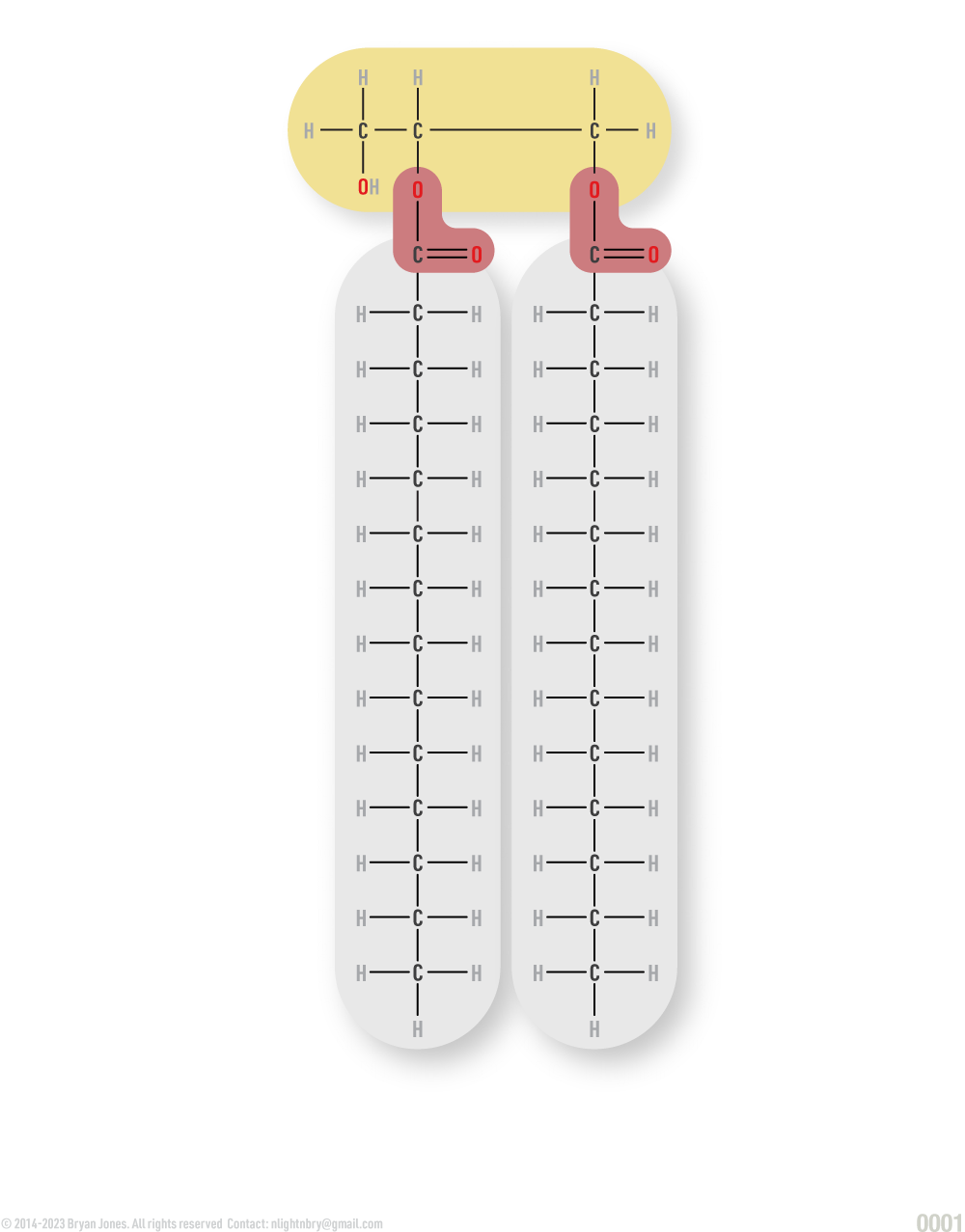
Triglyceride:
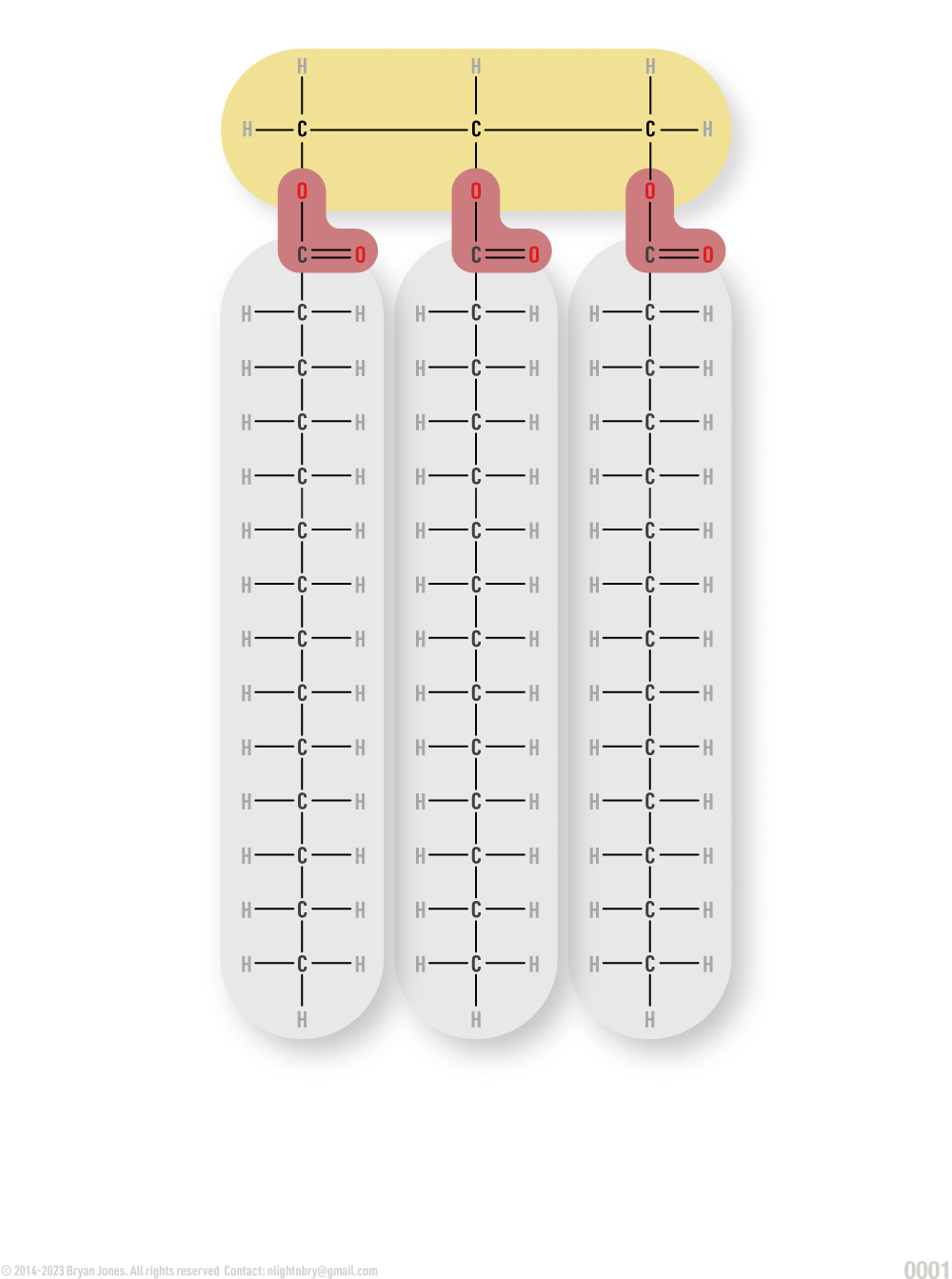
Complex Lipids
Complex lipids build cellular membranes.
Complex lipids build membranes, necessary to a cell’s survival. These complex lipids are called phospholipids. A cell is surrounded by phospholipids which act as a barrier between its water-based environment and its internal organelles.
Lipids contain both a fatty acid chain and glycerol. There are places on the glycerol where fatty acids attach in either one, two or three fatty acids clustered together. If a glycerol has two fatty acids attached it is referred to as a diglyceride and if it has three fatty acids, a triglyceride. Phospholipids consist of diglycerides and in place of a third fatty acid, a phosphate group.
Unlike simple lipids, certain phospholipids bear polar properties (meaning they have both an attractive and repelling facets). Other phospholipids are non-polar, or neutral. Like attracts like: nonpolar portions of phospholipids orient themselves toward nonpolar molecules. Polar portions of phospholipids can be hydrophobic or hydrophilic, orienting themselves away from, or toward, polar water molecules respectively.
Phospholipid Structure
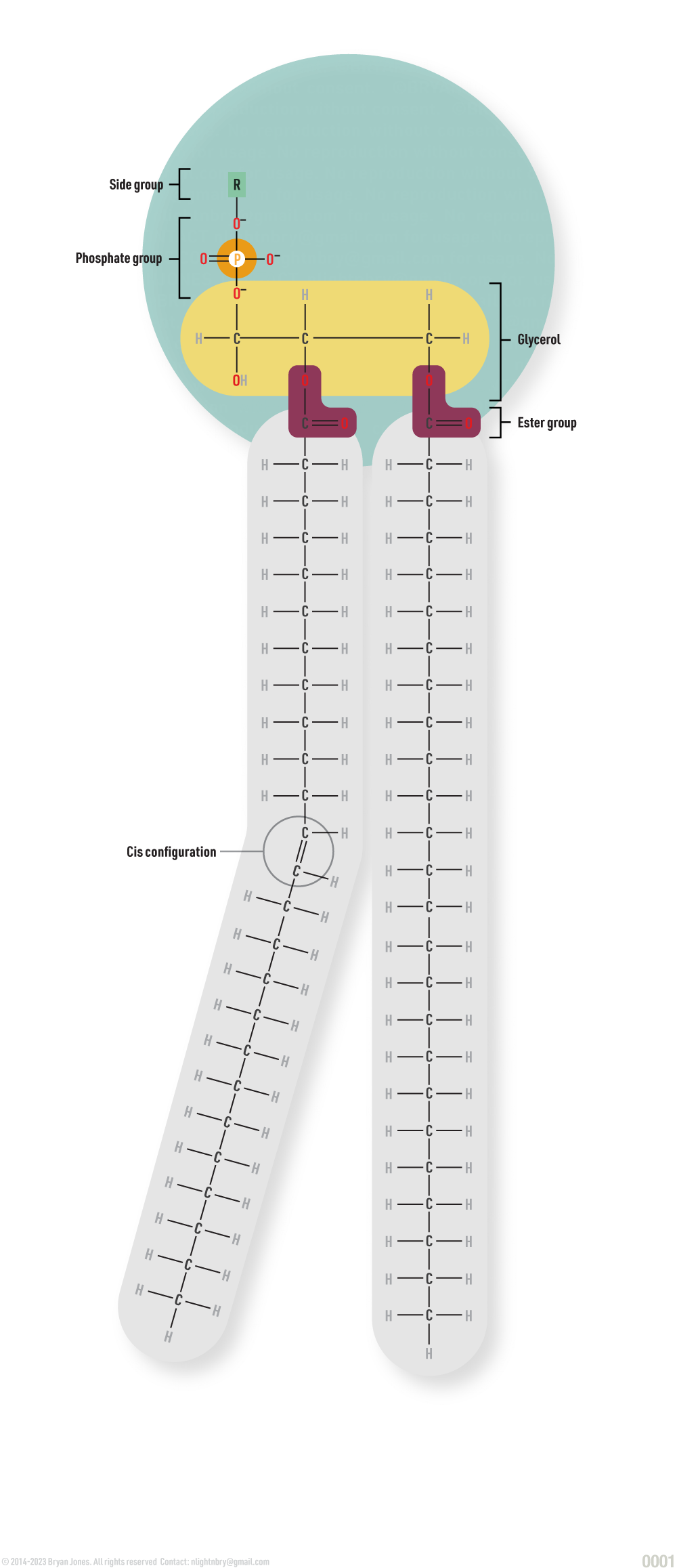
Simplified phospholipid graphic:

Cross section of a single layer of phospholipids:

Cross section of phospholipid bilayers:
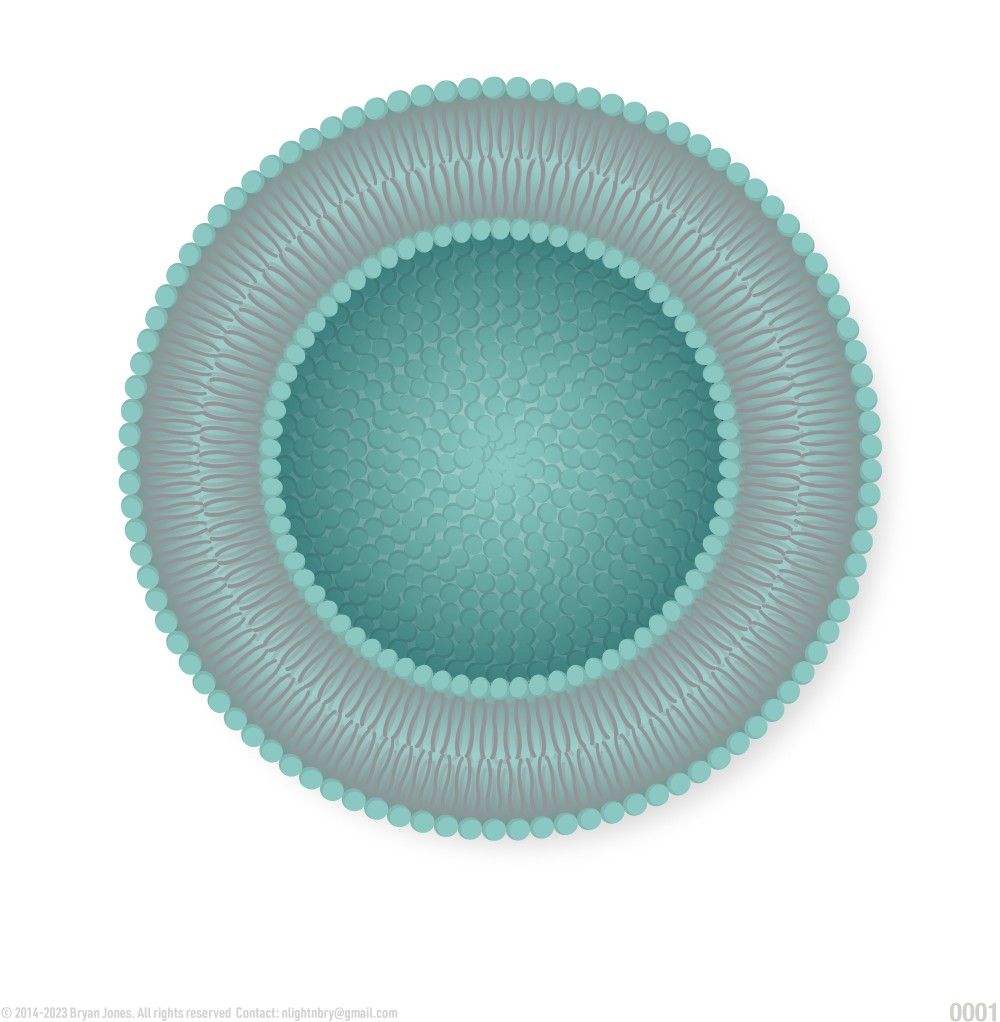
Thickness of phospholipid bilayer
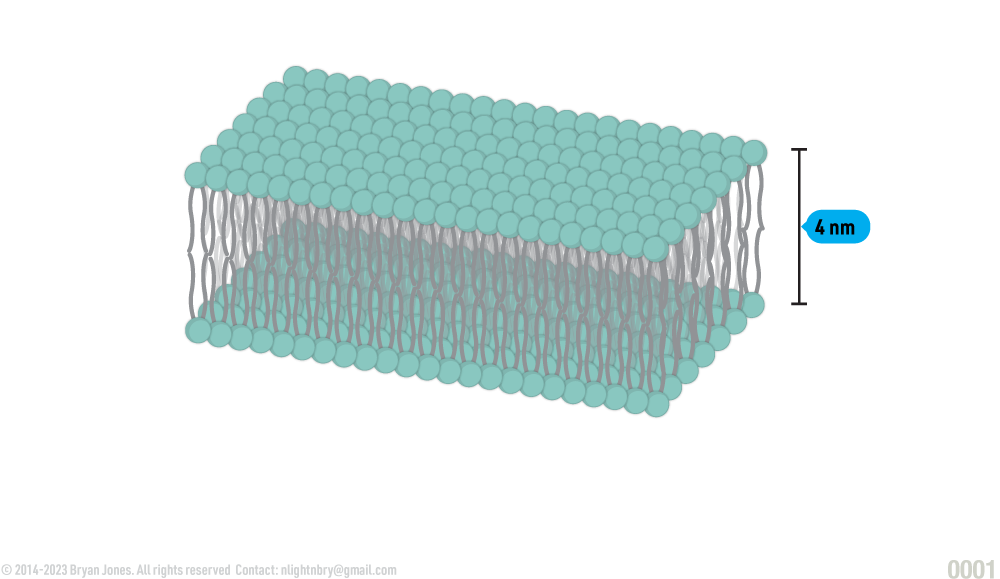
Orientation of phospholipids in a plasma membrane, where they form a bilayer, with the hydrophilic heads in contact with water and the hydrophobic tails oriented away from water:

Protein embedded within membrane

Complex Lipid Membrane
Lipid membranes act as a barrier that separates the cell's interior from its surroundings. This prevents the free diffusion of water and other molecules into and out of the cell, which is essential for maintaining the cell's internal environment.
Membrane Permeability
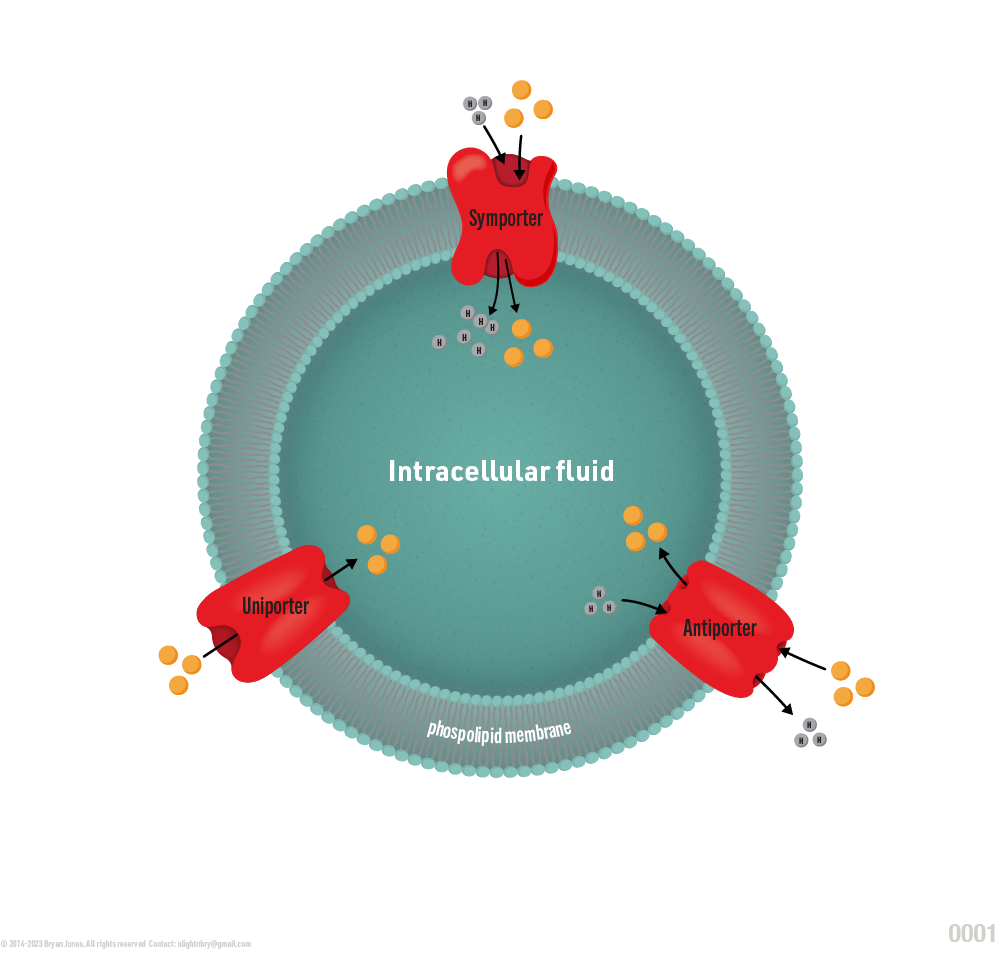
Passive transport and active transport are two different mechanisms that cells use to move molecules across their membranes. Passive transport does not require energy, while active transport does.
- In passive transport, molecules move down their concentration gradient, meaning that they move from an area where they are more concentrated to an area where they are less concentrated. This can happen through diffusion, osmosis, or facilitated diffusion.
- In active transport, molecules move against their concentration gradient, meaning that they move from an area where they are less concentrated to an area where they are more concentrated. This requires energy, which is usually provided by ATP. Active transport can occur through a variety of mechanisms, including carrier-mediated transport and vesicular transport.
Membrane Summary

Both passive and active transport are essential for the functioning of cells. Passive transport allows cells to take in nutrients and get rid of waste products. Active transport allows cells to maintain their internal environment and to transport molecules that are essential for cellular function.
"I'm tickled"

Membrane Transport

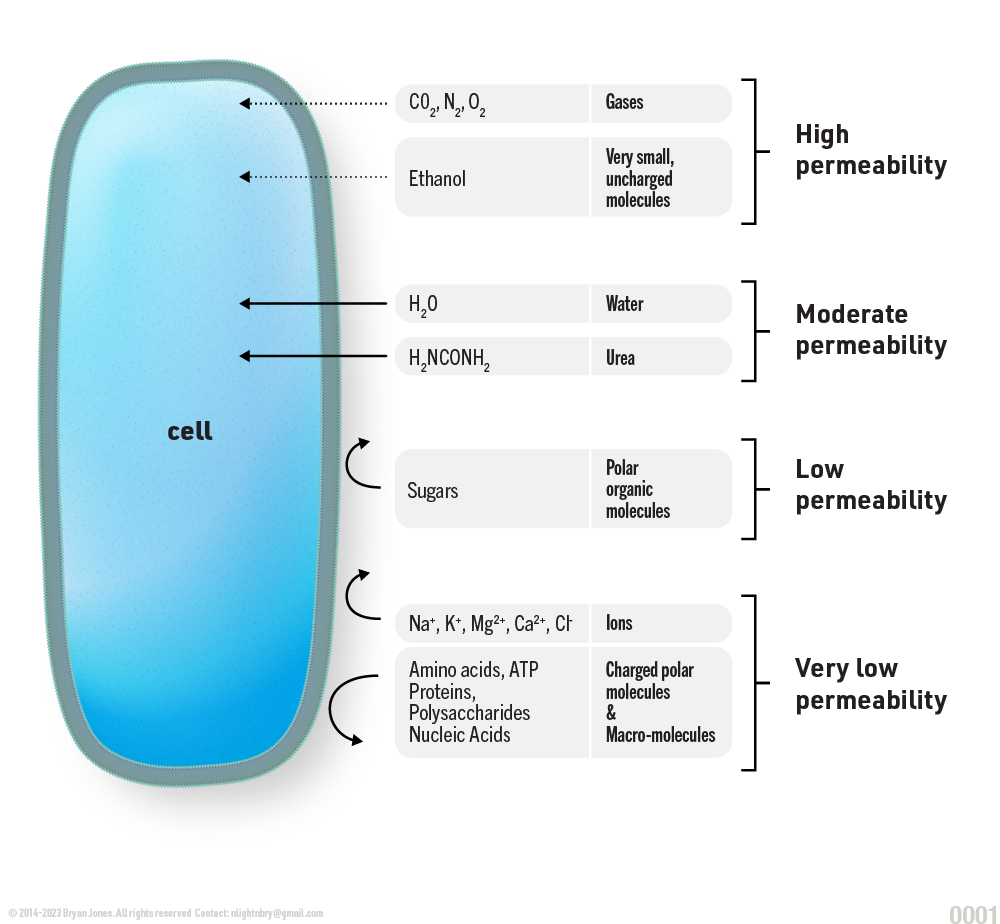
Why does HCl diffuse through membranes of stomach lining slower than H2O?
Why does Glucose have a permeability of 10^-3 while Tryptophan has a permeability of ~10^1?
To understand permeability and diffusion across a membrane it's important to consider coefficients.

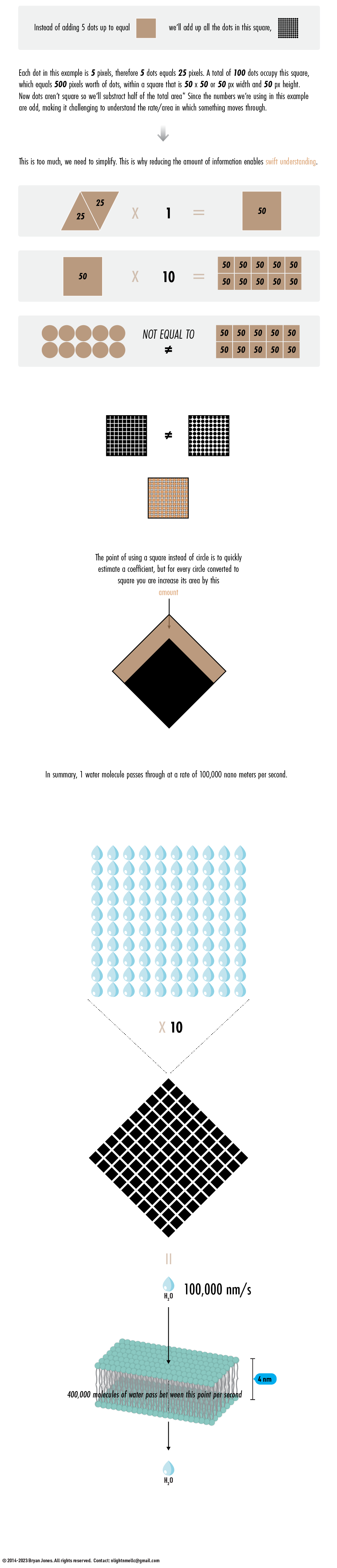
Q: What do these dots mean?
A: Think of them as a graphic representation of the rate or coefficient in which something can pass through. As you might expect, Water (H2O) diffuses across membranes very quickly, keeping your body healthy and lubricated. Water's permeable value of 10^5 enables reactions that use water to break bonds quickly. You don't want to use more energy just to make more energy. Water's ability to diffuse across membranes without having to use energy to transport it across membranes makes it useful, and it keeps your skin smooth. Skin elasticity is measurable: stretchy, squishy, flexible are all important variables to understand how a body works, when you feel "dry" it's due to dehydration, and/or something bright, emitting lots of heat, like a sun.
One reason why Alcohol dehydrates your body: Alcohol is a diuretic, which means it promotes increased urine production by affecting the body's hormone systems. When you drink alcohol, it inhibits the release of vasopressin, an anti-diuretic hormone. Vasopressin is responsible for regulating the body's water balance by controlling the amount of water reabsorbed by the kidneys.
With the suppression of vasopressin, the kidneys don't reabsorb as much water as they normally would. As a result, more water is sent to the bladder rather than being reabsorbed into the body. This process leads to increased urination. The more alcohol you consume, the more dehydrated you can become due to this increased urine production.

Circle Size (1:57)
(+) Wait.. so membrane permeability coefficient is why swallowing lots of water has kept me an independent healthy woman?
(–) Yes.
(–) You're getting the hang of it. You are filled with potential.
(+) Are you being salty with me?
(–) Depends on how long we're together.
(+) Sodium diffuses at 10-5 nm/s . You sure you want to make that joke?
(–) Na
(+) How original.
(–) Yes, and?
(+) ...
(–) Want some ice ... water, leaf, maybe a sun stone?
(+) Friendship gotta be hi for that to work.
(–) I'll give you fire, If you keep given me lip.
(+) You got any shinny stones?
(–) I tossed those.
(+) Yes, and?
Membranes are more permeable to uncharged compounds and least permeable to charged ions.
(–) Let me pin your hair back.
(+) As long as I get to pen you.
(–) You got enough ink?
(+) No, I got it all over my hands trying to figure it out.
(-) I'm going to eat your sandwich on this white counter top. Are those dusk stones?
(+) From this angle they are.
(-) I want some salt.
(+) Less talk more mumbles. Drink from these semi-permeable membranes.
(-) Delicatessen Chew-Ri is delicious.
(+) Beat history for me.
(-) Buns went to the floor.
(+) 5 second rule.
(-) It's been more than 5 seconds.
(+) Yes, and?
The disruption of neural signals during a forceful impact, such as a knockout punch, involves several mechanisms. While the exact details can be complex, the primary factors contributing to this disruption include rotational forces and the stretching of neural structures.
- Rotational Forces A powerful blow to the head can cause the head to rotate rapidly. The brain, suspended within the cerebrospinal fluid, may lag behind the movement of the skull. This differential movement creates shear forces that affect the neural tissues.
- Stretching of Neural Structures The brain is comprised of delicate neural tissue, including axons responsible for transmitting signals between neurons. The rapid rotation and movement of the head can lead to stretching and deformation of these neural structures. This stretching interferes with the normal electrochemical processes involved in transmitting signals along the axons.
- Axonal Disruption The axons, which conduct nerve impulses, can be damaged during this process. Axonal injury disrupts the flow of information, impeding the normal communication between neurons.
Gradient Potential it's important to note that neural signals involve the movement of ions across the cell membrane, creating changes in membrane potential. Disruption in this process, such as due to stretching or shearing forces, can affect the ability of neurons to generate and transmit action potentials.
(+) Yes, and?
(-) I'm going to drink this creamy latte.
(+) Make it a Grande.
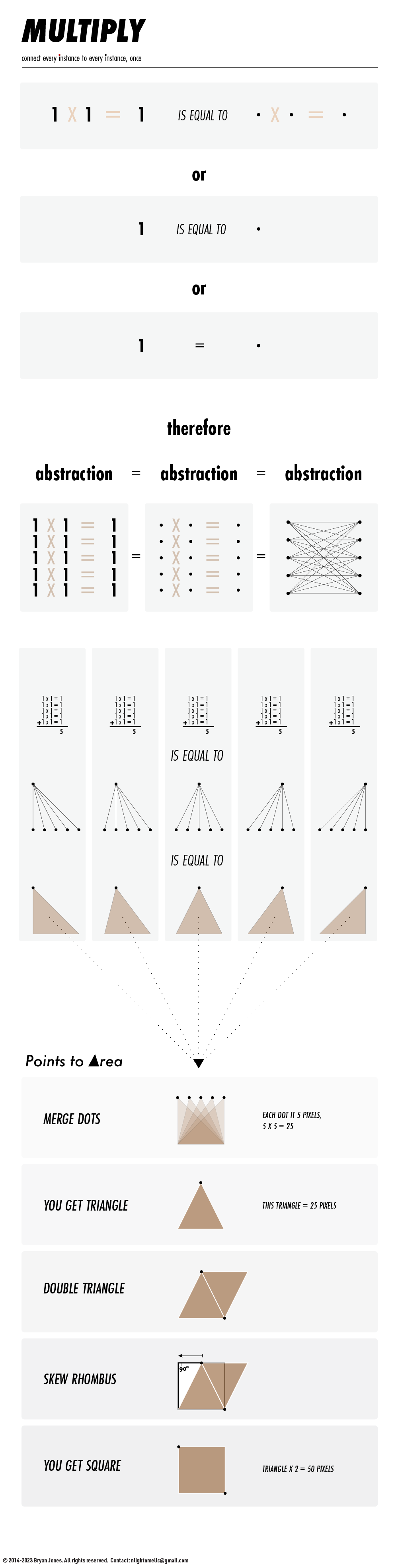
These dots are a slice of a pathway. The circumference of the dot, and the amount of dots (concentration), dictates the rate in which something can pass through, or the area in which something can travel. Converting numerical abstractions to geometric shapes as proof of concept has a long history.
YOU GET TRIANGLE
DOUBLE TRIANGLE
SKEW TIL YOU GET 90
GET SQUARE
"Tell our viewers about your outfit, your lips are gorgeous, can you tell us anything about them?"
"I use them to diffuse situations" -Woman

Wanting to brush up on your celestrial mechanics? Hands-on demonstrations are available, if welcomed by grande and snack. Otherwise, distance is how far it goes, velocity is how fast it's moving, and acceleration is how quick I can get it you up to speed.
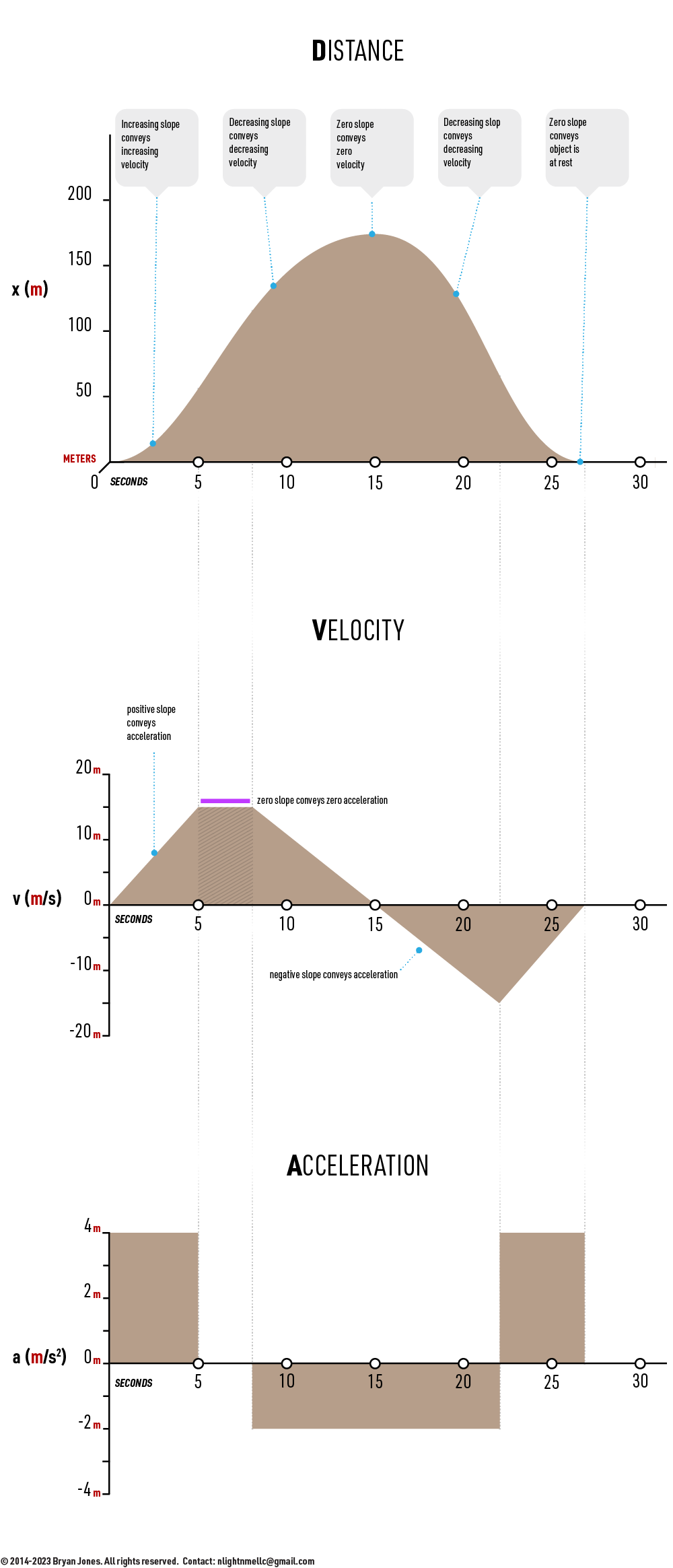
Test your understanding of ADVancement within Bonds and Forces page.
Attractive ♀ 👩🔬 welcome to ask questions and correct, unless malcious, then you can calculate the coefficent in which you pass through/in a 🕳.
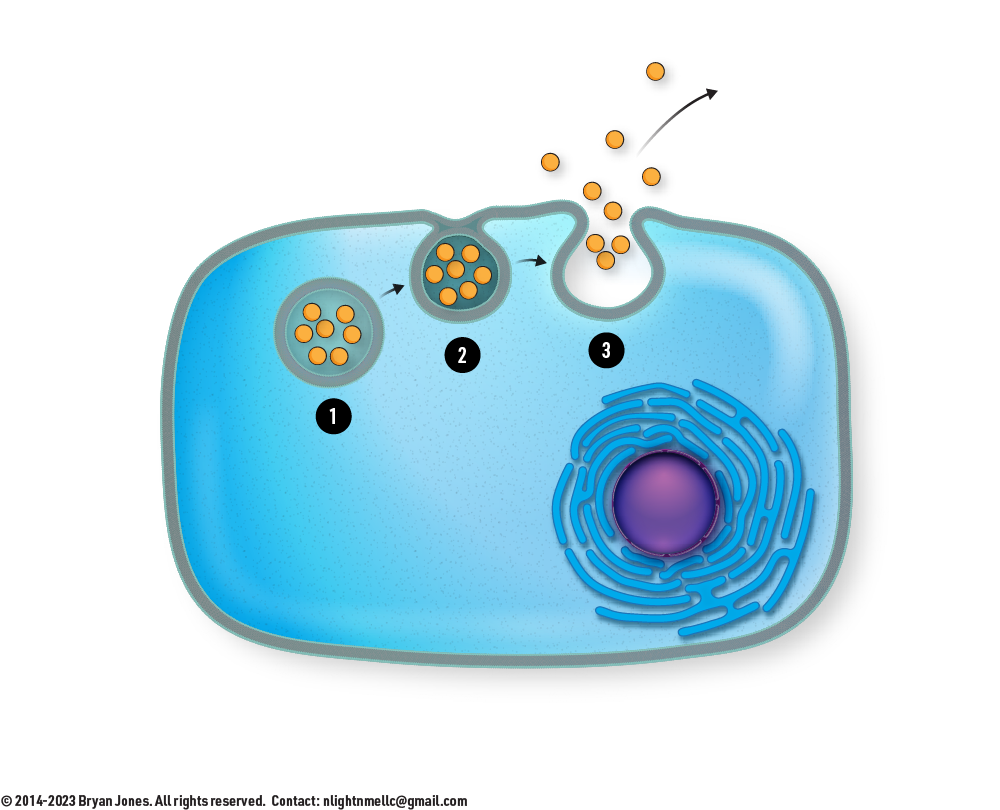
Endocytosis and Exocytosis
Endocytosis is when stuff is taken in by a cell, Exocytosis is when a cell pushes out internal material.
Endocytosis
- Initiation: The first step of endocytosis involves the recognition and binding of specific molecules or particles that the cell wants to internalize. This recognition often occurs through receptors on the cell membrane that interact with the target molecules. There are three primary types of endocytosis: phagocytosis (cellular eating), pinocytosis (cellular drinking), and receptor-mediated endocytosis. The specific method depends on the nature of the molecules being transported.
- Vesicle Formation: After the target molecules or particles bind to the cell membrane receptors, the cell membrane invaginates, forming a small, fluid-filled pocket called a vesicle. In phagocytosis, the vesicle is called a phagosome, and it engulfs solid particles, such as bacteria. In pinocytosis, the vesicles are called pinocytic vesicles and contain liquid or dissolved molecules. In receptor-mediated endocytosis, specific receptors are clustered in coated pits on the cell membrane, and when bound to their ligands, these coated pits invaginate and form coated vesicles.
- Vesicle Internalization: The final step is the internalization of the vesicle. The vesicle containing the engulfed substances or particles is pinched off from the cell membrane and released into the cell's cytoplasm. Once inside the cell, the vesicle may fuse with other cellular organelles, such as lysosomes, where the contents are digested or processed, depending on the nature of the materials.
"The following covers endo and exocytosis" -Ho son

Endocytosis

Exocytosis
- Vesicle Formation:The first step involves the formation of a vesicle within the cell. This vesicle, often called a secretory vesicle or exocytic vesicle, contains the substances that the cell intends to release. These substances can be various molecules, such as hormones, neurotransmitters, or cellular waste products. The vesicle is usually formed within the cell's Golgi apparatus or other organelles involved in the secretory process.
- Vesicle Transport:The second step is the transport of the vesicle containing the substances to the cell's plasma membrane. This transport is typically facilitated by motor proteins and the cytoskeleton. The vesicle is moved along microtubules to the cell membrane. During this transport, the vesicle may undergo maturation and fusion with other vesicles, modifying its contents and structure.
- Membrane Fusion and Release:The final step of exocytosis involves the fusion of the secretory vesicle with the cell's plasma membrane. This fusion allows the vesicle's contents to be released into the extracellular space. The plasma membrane and vesicle membranes merge, creating a pore through which the substances inside the vesicle are expelled into the surrounding environment. This release can be highly regulated and controlled, as in the case of neurotransmitter release at synapses, or it can be more constitutive, such as the secretion of digestive enzymes by pancreatic cells.
Exoocytosis
Prostaglandins
Prostaglandins, hormone-like chemical messengers, though they work locally within the cells where they are created.
Prostaglandins are a group of hormone-like molecules that play a wide range of roles in the body. They are produced in almost all tissues and act locally, meaning they affect cells near the site of production. Some of their functions include regulating inflammation, blood clotting, and the contraction and relaxation of smooth muscle tissue. They also have a role in regulating body temperature, pain perception, and the immune response. Additionally, prostaglandins are involved in female reproductive health, where they help regulate ovulation, cervical mucus production, and uterine contractions during childbirth. Overall, prostaglandins play a crucial role in maintaining homeostasis in the body.
Prostaglandins are unsaturated carboxylic acids, consisting of of a 20 carbon skeleton which also contains a five member carbon ring (pentose). There are a variety of structures one, two, or three double bonds. On the five member carbon ring there may also be double bonds, a ketone, or alcohol groups.
Prostaglandins are special chemicals that are made by your body to help with different processes. They can affect things like pain, inflammation, and even fever. When you get hurt or have an infection, your body sends out prostaglandins to help you feel better. They can cause swelling and redness in the area of your injury or infection, which might not feel good, but it's actually your body's way of trying to heal itself. Sometimes, prostaglandins can also cause cramps during your period or help your body start giving birth when it's time for a baby to come out. So, prostaglandins are like little messengers in your body that help with all kinds of important things!
Structure of a Prostaglandin

Steroids
Steroids are lipids but with a completely different structure than any other lipid. They work in conjunction with other lipids to prevent the hardening of plasma membranes. The best known of these is the sterol, meaning a steroid with an hydroxyl functional group (-OH) called cholesterol.
Steroids are a type of lipid molecule that play a crucial role in various physiological processes. They function as signaling molecules, regulating gene expression and controlling metabolism, growth, and development. They are also involved in maintaining the integrity of cell membranes and influencing the immune system. Steroids can be produced naturally by the body or synthetically, and are commonly used in medicine to treat conditions such as inflammation, allergies, and hormone imbalances. However, abuse of synthetic steroids can have serious negative effects on health, including liver damage, infertility, and cardiovascular disease.
When all you can do is ster

Steroids are characterized by their four interconnected carbon rings, found in animal hormones and cell membranes. Sterols are a type of steroid with an alcohol or hydroxyl functional group attached - specifically, cholesterol. At low temperatures, fatty acid chains pack together stiffly - sterols create a spaces to separate the plasma membrane, thus preventing hardening.
This is cholesterol, a steroid:

Testosterone

Estrogen

Waxes
A slightly different type of lipid structure
Biological waxes are a type of lipid found in many organisms, including plants and animals. They are composed of long-chain fatty acids and alcohols, and their function varies depending on the organism. In plants, waxes help to reduce water loss and protect the plant from environmental stresses such as UV radiation, insects, and pathogens. In animals, waxes can serve as a protective coating on feathers, fur, or skin, or they may be involved in waterproofing, such as in the waxy secretion on the surface of mammalian skin. Waxes can also play a role in communication, such as in the production of pheromones by insects and other animals.
A wax is a chemical functional group, known as an ester group, formed between a long chain alcohol and a saturated fatty acid. The characteristics of a wax are pliability, easily malleable when warmed and brittle and water resistant when cold. Many living things (from vegetables to human beings) have a wax film on their skin. This helps manage the gaining and losing of water and other compounds.
Structure of a wax:

"We're bonding, say Ah?" -Me

The same structure as above:

Lipid Comparison
Fatty acyl, phenol lipid, sphingolipid, glycerolipid, glycerolphospholipid, saccharolipid
Lipid Comparison

Lipid Oxidation
Lipid oxidation specifically refers to the oxidative degradation of fats, while carbon oxidation is a more general term encompassing the oxidation of carbon atoms in various chemical and biological processes.
Lipid oxidation refers to the process of oxidation or degradation of lipids (fats) in the presence of oxygen. It is a chemical reaction that involves the breakdown of the lipid molecules, leading to the production of free radicals and various oxidation products. This process commonly occurs in foods containing fats, such as oils, meats, and dairy products, and it is a major cause of food spoilage and quality deterioration.
Lipid oxidation typically involves three stages: initiation, propagation, and termination. During initiation, free radicals are formed by the action of oxygen, heat, light, or catalysts. These free radicals then propagate the reaction by reacting with nearby lipid molecules, leading to the formation of more free radicals. This chain reaction continues until termination, where the free radicals are either consumed or neutralized.
Lipid oxidation can result in the development of off-flavors, rancidity, color changes, and the loss of nutritional value in food products. It can also have negative effects on human health when oxidized lipids are consumed in excessive amounts. Antioxidants, such as vitamin E and certain food additives, are commonly used to inhibit or slow down lipid oxidation in food systems.
Carbon oxidation, on the other hand, is a broader term that refers to the process of carbon atoms combining with oxygen atoms or other oxidizing agents. It involves the transfer of electrons from the carbon atoms to the oxidizing agent, resulting in the carbon atoms losing electrons and undergoing oxidation.
Peppermint and spearmint contain menthol, which has been studied for its potential effects on lipid metabolism.
"Don't forget to breath ... want some spearmint?" -Man

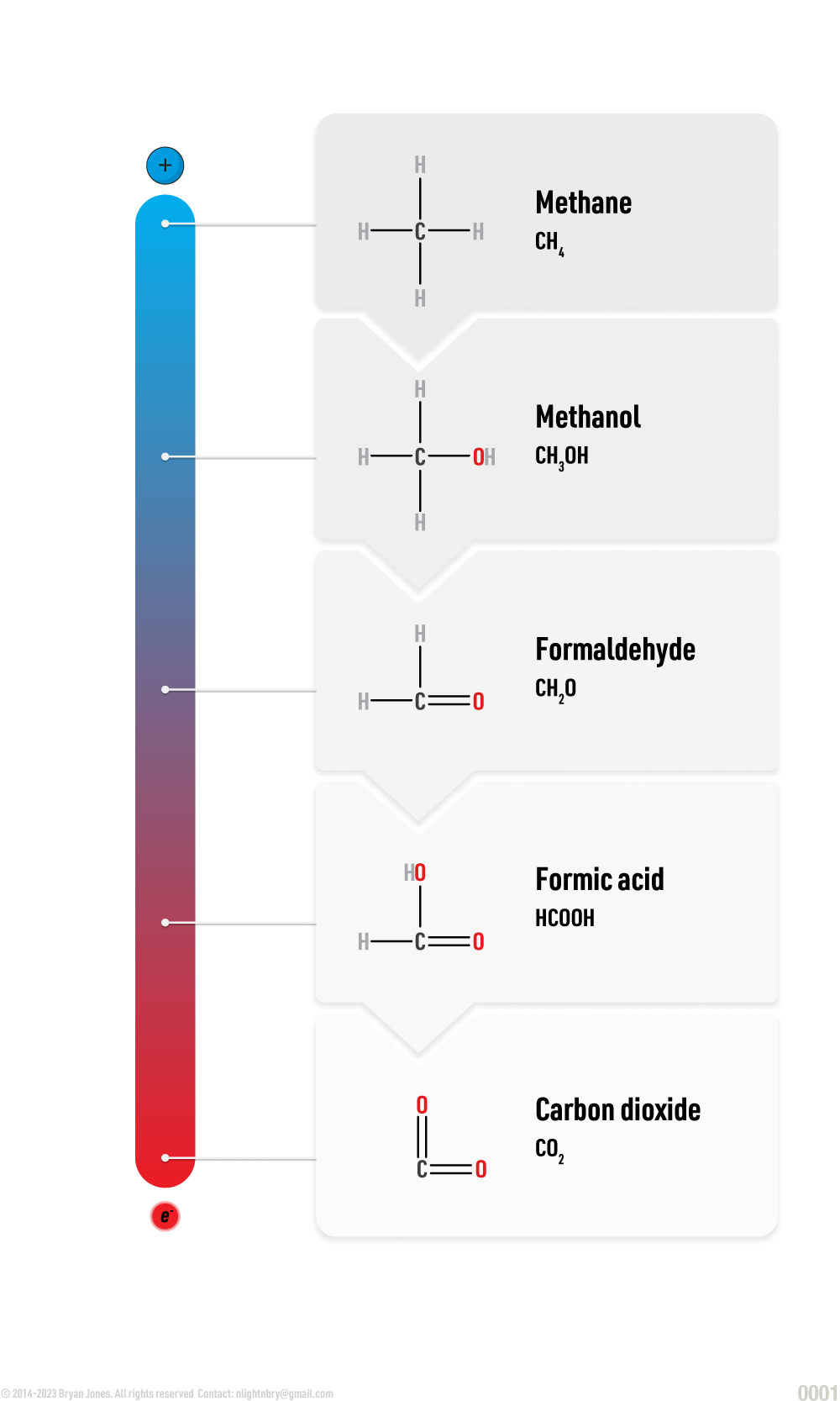
Carbon Oxidation
Lipids Summary
Lipids are a diverse group of biomolecules that play important roles in the structure, function, and energy storage of cells. They are hydrophobic, meaning they do not dissolve in water, and are characterized by their insolubility in water but solubility in nonpolar organic solvents. Lipids are important for a variety of functions in the body, including energy storage, insulation, and cell signaling. They are also involved in the absorption and transport of fat-soluble vitamins and play a role in the formation of hormones and cell membranes. However, an excessive intake of certain types of lipids can lead to health problems such as obesity, cardiovascular disease, and type 2 diabetes.
Contents
Lipid Catabolism
Breakdown of lipids into smaller molecules for energy
lipid catabolism is the process of breaking down lipids into smaller molecules, such as fatty acids and glycerol. These smaller molecules can then be used for energy production.
Graphic

Pathway Beta-Oxidation