Chemical Shorthand: Formula & Models
The atomic content of molecules can be represented by a few convenient formulas. When you think of Chemical Shorthand you should think about what is the easiest way to write the location of atoms in relations to each other. Their are many ways to graphically represent these atoms on a flat plane, but you soon will realize none of them are perfect.
Chemical Formula & Molecular Formula
Types of Chemical formula include: Empirical, Molecular, Structural, and Skeletal Formula
Empirical formula:
The empirical formula of an organic compound gives the simplest possible whole number ratio of the different types of atom within the compound. Similar to reducing a fraction.
Empirical formula gives the simplest possible whole number ratio, this is the girl at the bar that only answers yes or no, doesn't want to get into detail about her daily life, but she's aware of who sees her going home with. Similar to reducing a fraction or making a snap-judgments about this person's personality.
Molecular formula:
The formula concisely gives the atomic symbols and the number of the elements involved in subscript (CO2, H2O). Molecular formula only summarizes the atoms in a compound. they do not show the position of bonds between atoms. Molecular formulas provide a brief summary of the elements in a compound.
This is a basic meet up, no big deal, we're just chillin, we got our atomic symbols out their for everyone to see, we use subscript notation (CO2, H2O) to keep every thing hangin low, don't need any random erections to disrupt our understanding of the Carbon attached to 4 hydrogens, and Oxygen although technically speaking if we were to take a deeper look at this arrangement we'd see it's a ménage à trois plus Oxygen: a women with a strap-on with Hydrogen attached to her backend.
Molecular Formula

Displayed Formula
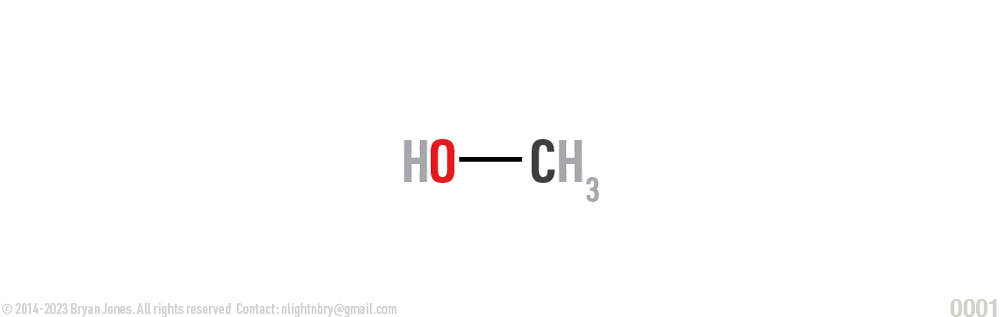
Structural formula:
Used to illustrate the relationships of the atoms and the number and types of bonds. Structural formulas clarify the exact relationships of the atoms in the molecule, depicting single bonds by a single line and double bonds by two lines.
When one person in the relationship asks "what are we" and you respond with we're in an exclusive relationship no cheating.
Structural Formula
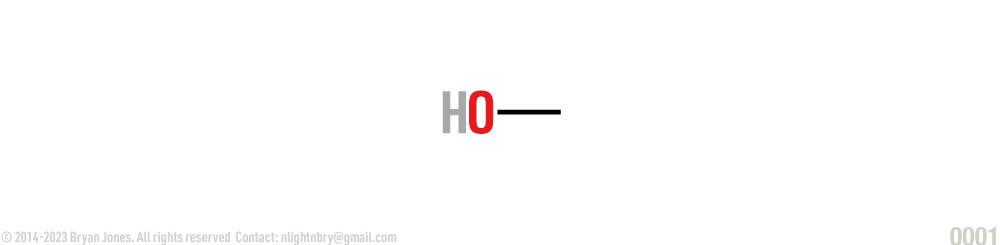
Skeletal formula:
In skeletal formula functional groups and atoms other than carbon or hydrogen are shown. Most hydrogens are omitted, because hydrogens are always connected to a carbon unless specified. Line ends or vertices represent carbons.
In skeletal formula you have a HO which is perfect for this example because carbon is pretty much a slut while hydrogen is being needy because hydrogen hasn't had any meaningful relationships other than quick meets. Most hydrogens are omitted because we forget about the needy people... atoms and we always know carbon has some side dude named hydrogen connected unless specified. At the end of the line there's a carbons, it's pretty much a penis in this example.
Skeletal formula:
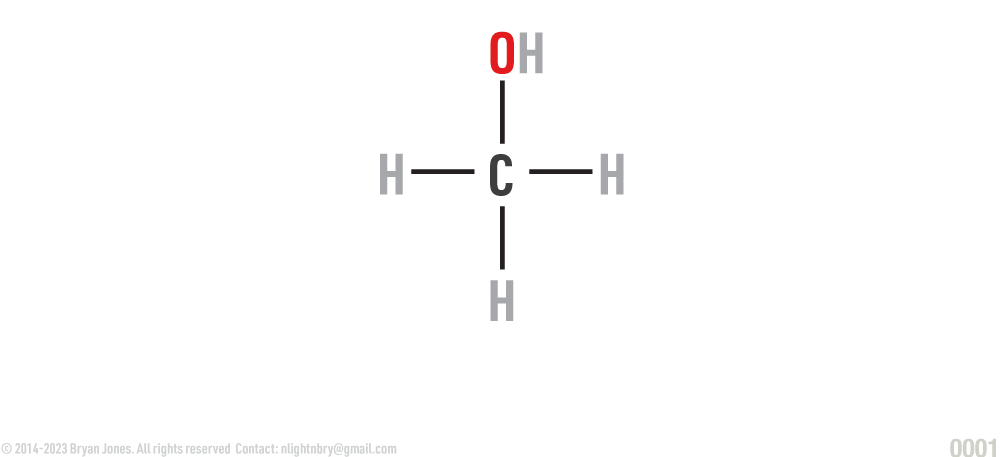
Molecular model: Ball and stick:
Shows geometry of a molecule. Atoms are represented as spheres and the sticks represent bonds.
Shows the dick-to-atom angle, atoms are spheres (girls have guys names too) and the sticks are obviously dicks.
Ball and stick model:
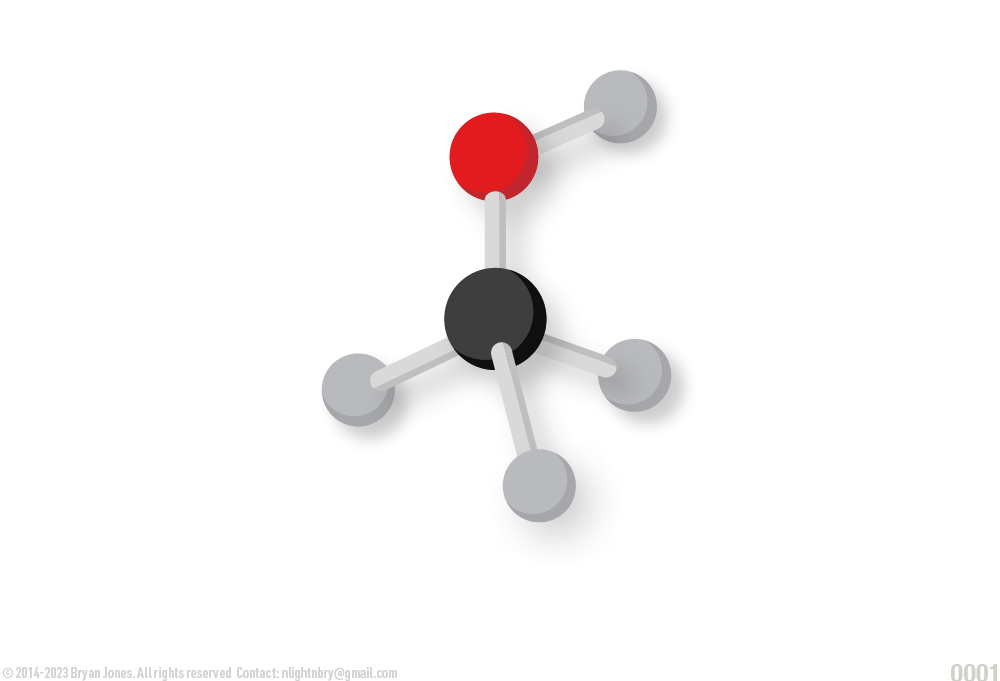
Space-filling model:
The space-filling model shows approximately what we think the electron clouds (orbitals) would look like if we could see them. Shows relative size of atoms in a molecule. Space-filling models, shows a three-dimensional appearance of molecules, the orientation of atoms (differentiated by color) and the molecule’s overall shape.
The space-filling model shows approximately what we think the carbon queef cloud would look like if we could see them. Here we get rid of the dick sticks and mash everyone together because its a girl's night out. While your out with your friends you may notice the relative sizes of each other in your selfies, depending on how the night goes, you may find yourself in a different position. It's okay to end up with hydrogen, it's better than As (Arsenic) or Boron (B)
Space-filling model:

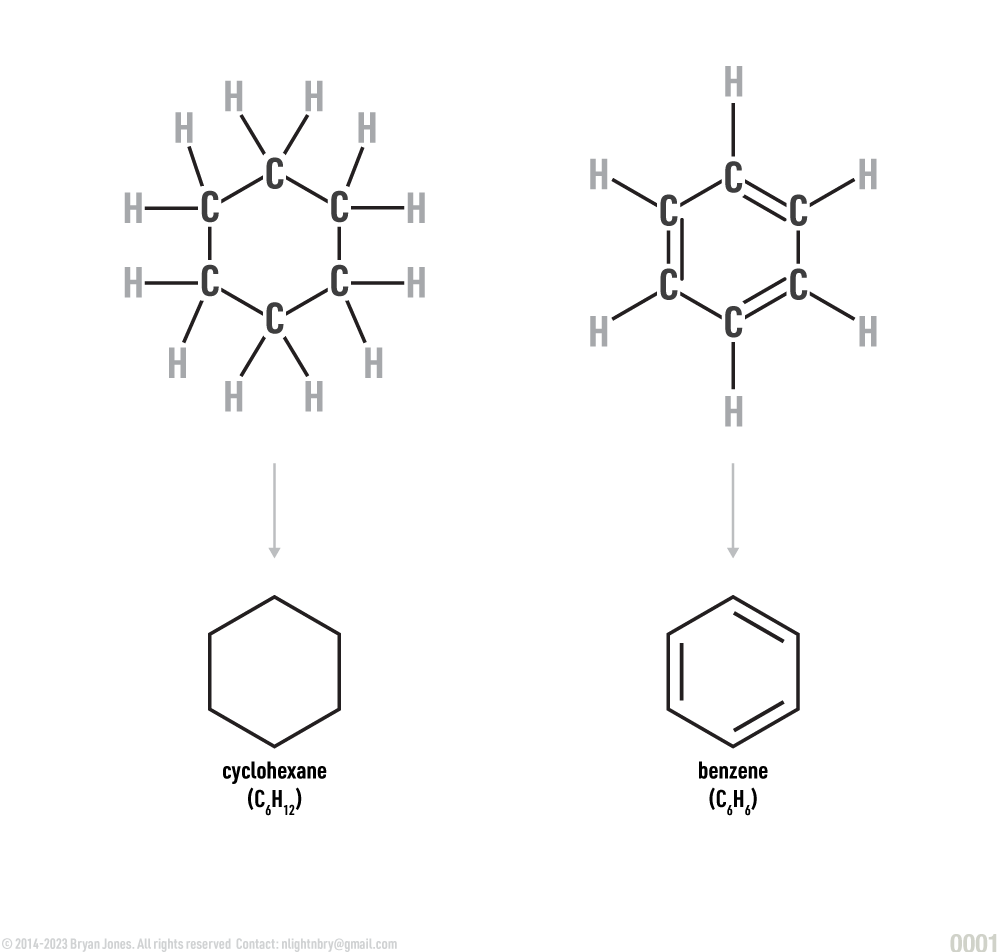
Structural Formula of Organic Compounds
Carbon at the angles
In structural formulas of organic compounds, cyclic or ringed compounds may be completely labeled, or they may be presented in a shorthand form in which carbons are assumed to be at the angles and attached to hydrogens.
Sometimes you want a ring other times you want to cycle around. This is the girl at the bar who has a ring on her finger and assume she's married.
Ways to represent carbon atoms:
Isomerism
Five main types of isomerism that can be exhibited by organic compounds
An isomer of a molecule is a molecule with the same molecular formula but a different structural or spatial arrangement of atoms. this variation can lead to a difference in physical or chemical properties.
This is a Kama sutra in a relationship, this is when trying every position possible.
Stereoisomerism
Optical

Non-superimposable mirror images of the same molecule
Optical isomers differ by the placement of different substituents, around one or more atoms in a molecule. Different arrangements of these substituents can be impossible to superimpose - these are optical isomers.
Geometic

Different substituents around a bond with restricted rotation
Commonly exhibited by alkenes, the presence of two different substituents on both carbon atoms at either end of the double bond can give rise to two different, non-superimposable isomers due to the restricted rotation of the bond.
Structural Isomerism
Functional

Different arrangement of a molecule’s carbon skeleton
The positions of the carbon atoms in the molecule can be rearranged to give ‘branched’ carbon chains coming off the main chain. The name of the molecule changes to reflect this, but the molecular formula is still the same.
Chain

The differing position of the same functional group in the molecule
The molecular formula remains the same; the type of functional group also remains the same, but its position in the molecule changes. The name of the molecule changes to reflect the new position of the functional group.
Position

Differing positions of atoms give a different functional group
Also referred to as functional group isomerism, these isomers have the same molecular formula but the atoms are rearranged to give a different functional group. The name of the molecule changes to reflect the new functional group.
Chirality
Methods for naming compounds
Information
FIGURE:

Naming Compounds
Available soon
Symbols - Greek
Symbols - Roman
Cation Monatomic Nomenclature
The nomenclature naming process for monatomic cations involves assigning names to positively charged ions formed by individual atoms. The naming convention generally follows a systematic approach based on the element's name and its oxidation state.
Here's a step-by-step explanation of the process:
- Identify the element: Determine the symbol or name of the element that forms the monatomic cation. For example, let's consider the element Sodium (Na).
- Determine the oxidation state: Find the charge or oxidation state of the monatomic cation. Monatomic cations always have a positive charge. In the case of Sodium, it forms a monatomic cation with a charge of +1.
- Determine the root name: The root name of the element remains unchanged. In the case of Sodium, the root name is "Sodium."
- Add the oxidation state: Use Roman numerals in parentheses after the root name to indicate the oxidation state. For Sodium with a +1 charge, we write "(I)" after the root name. The full name becomes "Sodium(I)."
Here are a few examples:
- Calcium (Ca) forms a monatomic cation with a +2 charge. The full name is "Calcium(II)."
- Iron (Fe) can form multiple monatomic cations with different charges. Iron(II) has a +2 charge, and Iron(III) has a +3 charge.
- Aluminum (Al) forms a monatomic cation with a +3 charge. Since Aluminum is in Group 3, we don't use Roman numerals. The name remains "Aluminum."
It's important to note that the nomenclature may vary for some transition metals, as they can exhibit multiple oxidation states. In those cases, Roman numerals are used to indicate the specific oxidation state.
Some elements can form multiple types of anions with different charges. In such cases, the charge is specified using Roman numerals in parentheses after the modified name. For example, iron can form both Fe2+ and Fe3+ cations, so the corresponding anions would be named ferrous (Fe2+) and ferric (Fe3+) respectively.
Some elements have fixed oxidation states and don't require the use of Roman numerals.
- Group 1 elements (Alkali metals) like Sodium always have a +1 charge, so the oxidation state is implied, and the name remains "Sodium" without any Roman numeral.
- Group 2 elements (Alkaline metals) like Magnesium always have a +2 charge, so the oxidation state is implied, and the name remains "Magnesium" without any Roman numeral.
Cation Polyatomic Nomenclature
The nomenclature naming process for polyatomic cations involves assigning names to positively charged ions that consist of multiple atoms bonded together. These ions typically have a net positive charge and behave as a single unit in chemical reactions.
Here's a step-by-step explanation of the process:
- Identify the composition: Determine the composition of the polyatomic cation by examining its chemical formula. Polyatomic cations are formed by groups of atoms bonded together. For example, let's consider the ammonium ion (NH₄⁺).
- Determine the root name: Identify the root name of the polyatomic cation based on the composition. In the case of the ammonium ion, the root name is "ammonium."
- Add the oxidation state: Polyatomic cations have a fixed net positive charge. In the case of the ammonium ion, it has a +1 charge. However, the oxidation state is typically not indicated in the name for polyatomic cations.
- Naming exceptions: Some common polyatomic cations have specific names that are widely recognized and used. These exceptions should be memorized separately. For example:
- H₃O⁺ is called the "hydronium ion."
- OH⁺ is called the "hydroxonium ion."
- NH₃⁺ is called the "ammonium ion."
It's important to note that polyatomic cations may have different oxidation states, which can affect their names. In those cases, the oxidation state is indicated with Roman numerals in parentheses after the root name. However, this is less common compared to monatomic cations.
Remember to consult reliable chemical references and guidelines for specific polyatomic cations, as there are exceptions and variations to the naming rules based on the specific composition and charge of the ion.
Anion Monatomic Nomenclature
The nomenclature naming process for monatomic anions involves assigning systematic names to negatively charged ions formed by individual atoms. These names are based on the element from which the anion is derived.
Here's a step-by-step explanation of the process:
- Identify the element: Determine the element that forms the monatomic anion. For example, if the anion is derived from oxygen, it would be an oxygen anion.
- Determine the charge: Determine the charge of the anion. Monatomic anions typically have a negative charge. The charge is usually indicated by the oxidation number or valence of the element. For example, oxygen typically has an oxidation number of -2, so the oxygen anion would have a charge of -2.
- Modify the element name: Modify the name of the element to reflect its anionic form. In general, the ending of the element name is changed to "-ide." For example, the oxygen anion becomes the oxide ion.
- Combine the charge and modified name: Combine the charge and modified name of the anion to form the final name. The charge is indicated as a superscript immediately following the element symbol or modified name. For example, the oxide ion with a charge of -2 is written as O2-.
Here are a few more examples:
- Chlorine anion: Chlorine typically has an oxidation number of -1, so the chlorine anion is called chloride (Cl1-).
- Nitrogen anion: Nitrogen typically has an oxidation number of -3, so the nitrogen anion is called nitride (N3-).
- Sulfur anion: Sulfur typically has an oxidation number of -2, so the sulfur anion is called sulfide (S2-).
Anion Polyatomic Nomenclature
The nomenclature naming process for polyatomic anions involves assigning systematic names to negatively charged ions that consist of multiple atoms bonded together. These anions have specific names based on their composition and charge. Here's an explanation of the process:
Here's a step-by-step explanation of the process:
- Identify the composition: Determine the atoms present in the polyatomic anion and their respective quantities. This information is essential for constructing the name accurately.
- Determine the charge: Determine the charge of the polyatomic anion. This charge is typically indicated by the overall number of electrons gained or lost by the ion. The charge is crucial in naming the anion correctly.
-
Modify the name based on the charge: Depending on the charge of the polyatomic anion, modify the name to reflect the charge. There are two scenarios:
-
If the anion has a negative charge: The name typically ends with "-ate" or "-ite." The "-ate" ending is used for the higher charge or more oxygenated form, while the "-ite" ending is used for the lower charge or less oxygenated form. For example:
- NO3-: Nitrate
- NO2-: Nitrite
- SO42-: Sulfate
- SO32-: Sulfite
-
If the anion has a positive charge: The name is modified with the word "hydrogen" or "dihydrogen" to indicate the presence of hydrogen ions. The charge is also indicated using Roman numerals in parentheses. For example:
- HCO3-: Hydrogen carbonate or bicarbonate
- H2PO4-: Dihydrogen phosphate
- NH4+: Ammonium
-
If the anion has a negative charge: The name typically ends with "-ate" or "-ite." The "-ate" ending is used for the higher charge or more oxygenated form, while the "-ite" ending is used for the lower charge or less oxygenated form. For example:
-
Handle common polyatomic anions: Some polyatomic anions have well-established names that do not follow the "-ate" or "-ite" convention. These names are widely used in chemistry and are often memorized. Some common examples include:
- CO32-: Carbonate
- SO42-: Sulfate
- PO43-: Phosphate
- OH-: Hydroxide
- CN-: Cyanide
-
Consider additional elements: In some cases, the polyatomic anion may contain additional elements. The names of these elements are included within the anion's name to indicate their presence. For example:
- ClO4-: Perchlorate (contains chlorine and oxygen)
- ClO2-: Chlorite (contains chlorine and oxygen)
It's important to note that memorizing common polyatomic anion names is useful as they have specific names that are widely recognized and used in chemical nomenclature.
Examples of polyatomic anions:
It's also worth mentioning that there are some common polyatomic anions (anions consisting of multiple atoms) that have specific names, such as carbonate (CO32-), sulfate (SO42-), and phosphate (PO43-). These anions have well-established names that are widely used in chemistry.
- CO32- is called the "carbonate ion."
- NO3- is called the "nitrate ion."
- SO42- is called the "sulfate ion."
- PO43-is called the "phosphate ion."






