Electronegativity
Electronegativity is the attraction between electrons in an atom. Think of it as some atoms attract more electrons than others. Chlorine attracts electrons so well it's often used to break covalent and ionic bonds. This is why bleach is used to sanitize.
Electronegativity is a measure of how strongly an atom attracts electrons towards itself when it forms a chemical bond with another atom. Atoms with a high electronegativity tend to "hog" the shared electrons in a bond, while atoms with a low electronegativity tend to share the electrons more equally.
Chlorine is an atom with a high electronegativity, which means it attracts electrons very strongly. This makes it a very reactive element, and it can be used to break apart or "oxidize" other compounds. For example, bleach contains chlorine and can be used to sanitize surfaces by breaking down organic compounds and killing bacteria.
When chlorine is used in chemical reactions, it can sometimes break covalent and ionic bonds because it attracts electrons so strongly. In an ionic bond, like the one between sodium and chloride in table salt (NaCl), chlorine's strong electronegativity attracts the electron in sodium's outermost shell, creating a positively charged sodium ion and a negatively charged chloride ion.
In a covalent bond, which is when two atoms share electrons to form a molecule, chlorine's electronegativity can create a polar bond, where one end of the molecule has a partial negative charge and the other end has a partial positive charge. This can make the molecule more reactive and able to break apart other compounds.
So, in summary, electronegativity is a measure of how strongly an atom attracts electrons, and chlorine's high electronegativity makes it a powerful and reactive element that can break down other compounds in chemical reactions.
Electronegativity is how attractive the person is in front of you, if you're willing to risk rejection, then they must be pretty attractive. Some people are more attractive than others and some are very attractive like Chlorine, They'll take your soul.
Electron Affinity
Electronegativity is the measure an atom’s attraction of electrons. It is the strength with which an atom holds electrons to itself. The higher the electronegativity, the more polar the bond the atom can form.
Difference in Electronegativity:


In a nonpolar bond, when two atoms have equal electronegativity, atoms have the same tendency to attract the bonding pair of electrons. To get a bond like this would require two of the same atom.
Theres two people in front of you and they're attractive, you think to yourself would I fuck them? No I have more self-respect than last weekend. Then you find out this is your sole mate, this is Hydrogen realizing they're attracted to another hydrogen and is okay with being a nonpolar covalent bond (H2), or when that slut Carbon is thinking to herself, it would be fun to know what two Oxygen's with strap-ons would entail (CO2), turns out it requires lots of energy to break the bonds between two strap-ons. Plus, hoe, and slut is an archaic term used to suppress autonomy. Witches used to get burned, now they're celebrated.

In a polar bond, in which there is a difference in charges between atoms. One atom has more electrons and so becomes slightly negative while the other atom becomes slightly positive. The resulting molecule is held together with a covalent bond but has sides which differ slightly in their charges. For example, H20 molecules are polar (oxygen has a negative charge while hydrogen has a positive charge).
You go to a bar, later you find yourself with lunch in you, because you were or are really attracted to the person this doesn't make you less of a person, this makes you more of a person, you can count on Hydrogen.

In a ionic bond, one atom’s electrons have been pulled away to the other atom giving that atom control over both electrons. This creates ionic bonds or ions.
Remember the fuck-boy from last weekend?
NO? well guess what you just found another, and you have excitement all over your face you can't help but smile thinking this guy really respects me, and you look over to your friends and you see they're talking to someone so your like well I'll just keep talking to him there's nothing slutty about that, then your like Oh I guess I'll just get naked and see what happens. He might not be a fuck-boy after all.
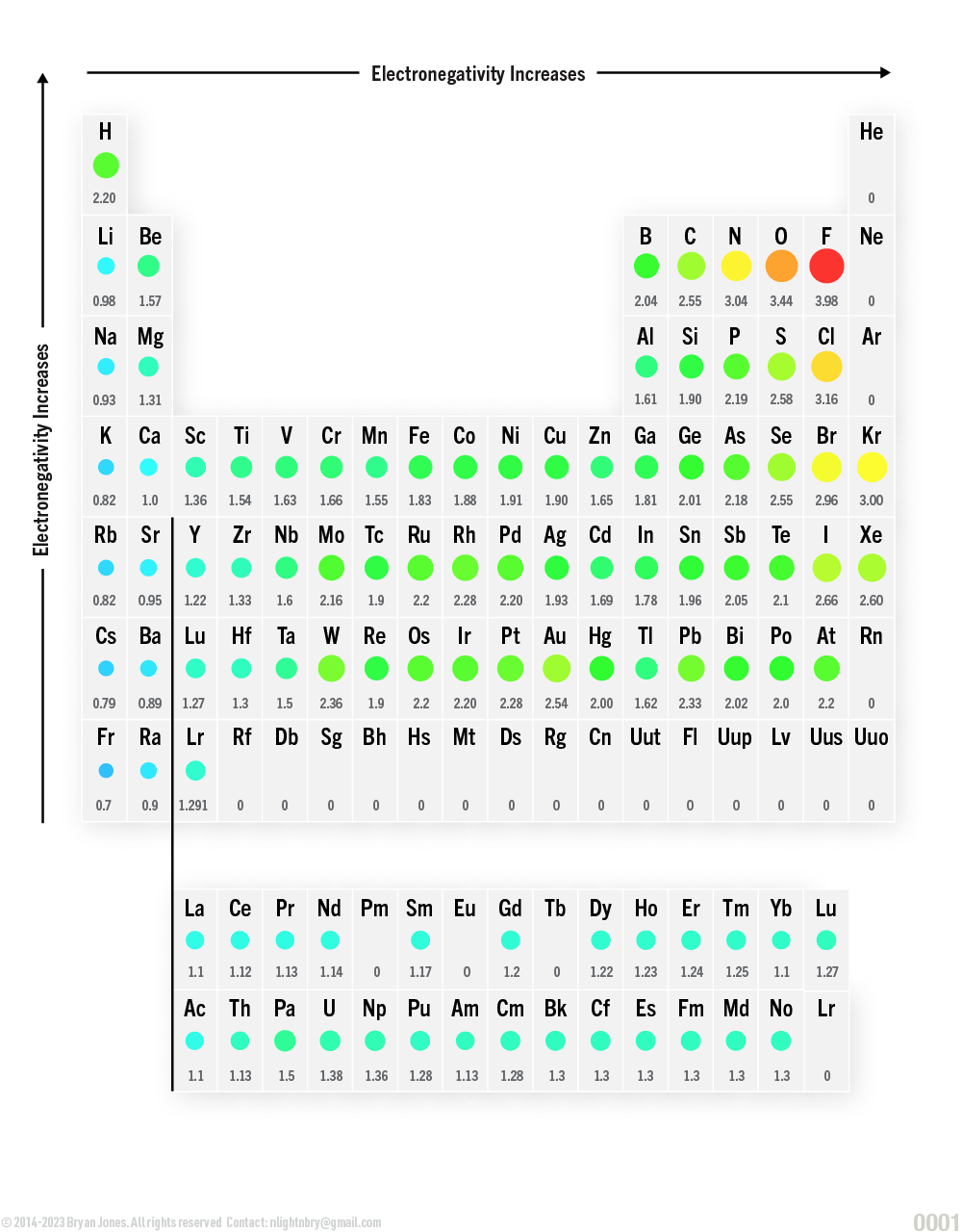
Table of Electronegativity
From left to right across the periodic table, the electronegativity of elements increase.
The metals at the left are more likely to lose electrons to achieve a stable electron configuration, so they are less likely to attract electrons. Elements on the right side of the periodic table tend to gain electrons in order to achieve a stable electron configuration. Because of this, these elements are more likely to attract electrons. Noble gases (furthest to the right) don’t have an electronegativity value because they don’t form bonds—they have a stable electron configuration.
Well this table should be easy to understand, blue are the male or females you won't have any luck with unless your putting forth energy, and the red are those that your likely already attached to or you can't get the fuck away from such as Fluorine (F).
Periodic Table:
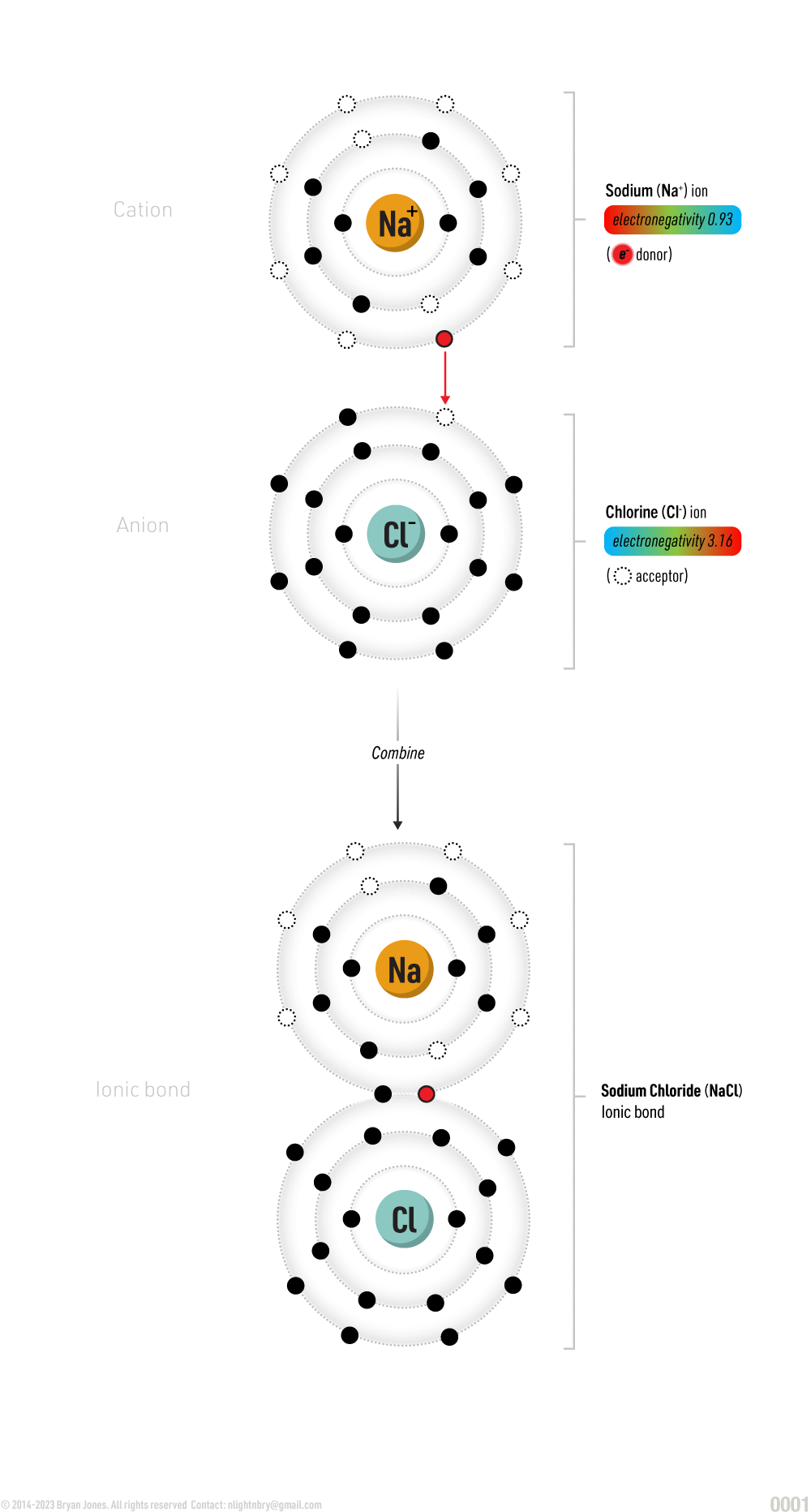
Two atoms with extremely different electronegativities:
In the case of sodium chloride (NaCl), the electron in sodium’s outermost shell has been pulled toward the chloride atom because that atom has a stronger electronegativity. Keep in mind that NaCl is an ionic bond, meaning that the electrons aren’t shared. Sodium has a positive charge, and the chloride atom has a negative charge. The larger the difference in electronegativity, the more negative and positive the atoms become, thus creating a polarized molecule.
everything in the world is made up of tiny building blocks called atoms. Sodium and chlorine are two different atoms that can combine to make a compound called sodium chloride, or table salt.
When sodium and chlorine come together to form a compound, they share their electrons with each other. But, in the case of sodium chloride, the electrons aren't actually shared evenly between the two atoms. Instead, the electron in sodium's outermost shell is pulled towards the chlorine atom because chlorine has a stronger attraction for electrons. This causes the sodium atom to have a positive charge and the chlorine atom to have a negative charge.
This difference in charge between the two atoms is what we call an "ionic bond". This means that the two atoms are attracted to each other because of their opposite charges. In the case of sodium chloride, the positively charged sodium atom is attracted to the negatively charged chlorine atom.
Because of this unequal sharing of electrons, the molecule of sodium chloride becomes "polarized". This just means that one end of the molecule has a slightly positive charge, while the other end has a slightly negative charge.
So, the next time you sprinkle some salt on your food, remember that it's actually a compound made up of two different atoms that are attracted to each other because of their opposite charges!
Sodium is the woman that has been studying really hard for the last week and gets all dressed up to go out to a restaurant or bar. She accidentally bumps into a male Chloride, she says "hi" and he's like "hey" in a very attractive dick-whisperer way, then you have the taste of Peppermint or Cinnamon on your tongue.
Ionic bond: