Functional Group
A functional group is a section of a compound that is classified as a specific group of bound atoms. Functional groups help determine the reactions and connections that a compound may engage in.
-
Amino Acids
-
- Structure and classification of amino acids
- Properties of amino acids
- Essential and non-essential amino acids
- Polar and non-polar amino acids
- Acidic and basic amino acids
- Amino acid sequencing
- Peptide bond formation
-
-
Protein Structure and Function
-
- Primary, secondary, tertiary, and quaternary structure of proteins
- Protein folding and stability
- Denaturation of proteins
- Protein-protein interactions
- Enzymes and catalysis
- Enzyme kinetics
- Allosteric enzymes
- Protein domains and motifs
-
-
Protein Synthesis
-
- Transcription and translation
- Genetic code
- tRNA and aminoacyl-tRNA synthetases
- Ribosomes and ribosomal RNA
- Protein targeting and transport
- Post-translational modifications of proteins
-
-
Proteomics
-
- Proteome and proteomics
- Two-dimensional gel electrophoresis
- Mass spectrometry
- Protein-protein interaction analysis
- Structural proteomics
-
-
Protein Engineering
-
- Rational protein design
- Directed evolution
- Protein expression systems
- Protein purification techniques
- Biophysical techniques for protein characterization
-
-
Diseases Related to Proteins
-
- Protein misfolding diseases
- Amyloidosis
- Prion diseases
- Proteopathies
- Protein aggregation
- Protein-losing enteropathy
-
Functional Groups of Organic Compounds:
Accessory molecules
Key characteristics of functional groups include: having consistent chemical properties and their atoms are linked to by covalent bonds. They behave as special accessory molecules which bind to organic compounds. The arrangement of a functional group makes it possible to predict how a new compound will react.
Characteristic Properties:
Specific groups of atoms
The bonding of other elements with carbon and hydrogen forms characteristic functional groups. Specific groups of atoms determine properties of a compound which are either chemical or physical. Chemical properties are used to characterize materials in reactions that change their identity. Physical properties are used to characterize matter and energy and their interactions.
The unique properties of an organic compound depend not only on its carbon skeleton but also on the atoms that are attached to the skeleton. In an organic molecule, the groups of atoms that usually participate in chemical reactions are called functional groups.
Functional Group Structure:
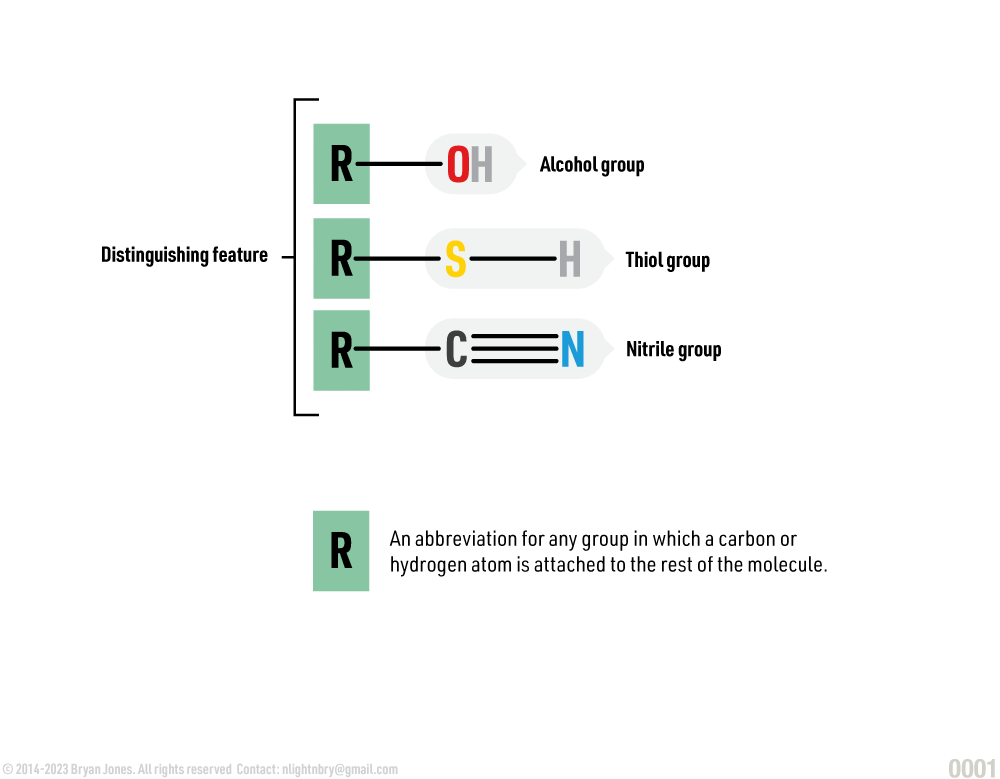
Table of Functional Groups:
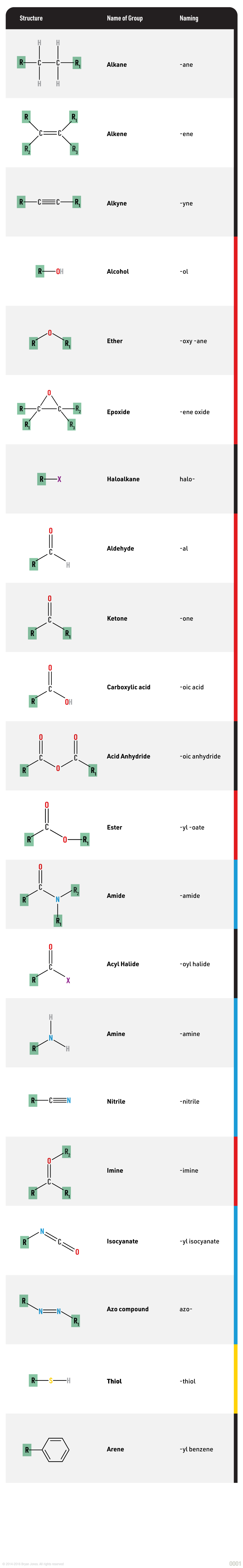
Hydrocarbons Functional Groups
| Chemical Name |
Group |
Formula |
Prefix |
Suffix |
Example |
Structural Formula |
|---|---|---|---|---|---|---|
| Alkanes | Alkane group | R-CH3 | Alkyl- | -ane | Ethane | CH3CH3 |
| Alkenes | Alkene group | R-CH=CH2 | Alkenyl- | -ene | Propene | CH3CH=CH2 |
| Alkynes | Alkyne group | R-C≡CH | Alkynyl- | -yne | Butyne | CH3C≡CH |
| Cycloalkanes | Cycloalkane group | CnH2n (n = number of carbon atoms) | Cycloalkyl- | -ane | Cyclohexane | (CH2)6 |
| Aromatic hydrocarbons | Aromatic group | Ar (Ar = aromatic ring) | Phenyl- | - | Benzene | C6H6 |
Oxygen Functional Groups
| Chemical Name |
Group |
Formula |
Prefix |
Suffix |
Example |
Structural Formula |
|---|---|---|---|---|---|---|
| Alcohols | Hydroxyl | ROH | Hydroxy- | -ol | Ethanol | CH3CH2OH |
| Aldehydes | Aldehyde | RCHO | - | -al | Formaldehyde | HCHO |
| Ketones | Ketone | R2C=O | - | -one | Acetone | CH3COCH3 |
| Carboxylic acids | Carboxyl | RCOOH | - | -oic acid | Acetic acid | CH3COOH |
| Esters | Carboxylate ester | RCOOR' | Alkyl/aryl- | -oate | Ethyl acetate | CH3COOCH2CH3 |
| Acid chlorides | Acyl chloride | RCOCl | - | -oyl chloride | Acetyl chloride | CH3COCl |
| Acid anhydrides | Acyl anhydride | (RCO)2O | - | -oic anhydride | Acetic anhydride | (CH3CO)2O |
| Ethers | Ether | R-O-R' | Alkoxy- | -ether | Dimethyl ether | (CH3)2O |
| Epoxides | Oxirane | O-CH2-CH2-O | - | -oxirane | Ethylene oxide | CH2CH2O |
| Peroxides | Peroxide | R-O-O-R' | Alkyl/aryl- | -peroxide | Hydrogen peroxide | H2O2 |
| Thiols | Thiol | R-SH | Mercapto- | -thiol | Ethanethiol | CH3CH2SH |
| Sulfides | Thioether | R-S-R' | Alkyl/aryl- | -sulfide | Dimethyl sulfide | (CH3)2S |
| Sulfoxides | Sulfoxide | R-S(=O)-R' | - | -sulfoxide | Dimethyl sulfoxide | (CH3)2SO |
| Sulfones | Sulfone | R-S(=O)2-R' | - | -sulfone | Dimethyl sulfone | (CH3)2SO2 |
| Acyl halides | Acyl halide | RCOX | - | -oyl halide | Acetyl chloride | CH3COCl |
| Carboxylic acid salts | Carboxylate | RCOOR' | Alkyl/aryl- | -oate | Sodium acetate | CH3COONa |
| Amides | Carboxamide | RCO-NH2 | - | -amide | Acetamide | CH3CONH2 |
| Nitro compounds | Nitro | R-NO2 | Nitro- | -nitro | Nitrobenzene | C6H5NO2 |
| Phenols | Phenolic hydroxyl | Ar-OH (Ar = aromatic ring) | Hydroxy- | -ol | Phenol | C6H5OH |
Nitrogen Functional Groups
| Chemical Name |
Group |
Formula |
Prefix |
Suffix |
Example |
Structural Formula |
|---|---|---|---|---|---|---|
| Amines | Amino group | R-NH2 | Amino- | -amine | Ethylamine | CH3CH2NH2 |
| Amides | Carboxamide group | RCO-NH2 | - | -amide | Acetamide | CH3CONH2 |
| Nitriles | Cyanide group | RC≡N | - | -nitrile | Acetonitrile | CH3CN |
| Nitro compounds | Nitro group | R-NO2 | Nitro- | -nitro | Nitrobenzene | C6H5NO2 |
| Azides | Azide group | R-N3 | Azido- | -azide | Phenyl azide | C6H5N3 |
| Imines | Imine group | R2C=NR' | - | -imine | Methylamine | CH3NHCH3 |
| Pyridines | Pyridine group | C5H5N | - | -pyridine | Pyridine | C5H5N |
| Quaternary ammonium salts | Quaternary ammonium group | R4N+ | - | -onium | Tetraethylammonium bromide | (C2H5)4NBr |
| Hydrazines | Hydrazine group | N2H4 | - | -hydrazine | Hydrazine | N2H4 |
| Oximes | Oxime group | R-C=N-OH | - | -oxime | Acetoxime | CH3C=N-OH |
| Imides | Imide group | R-CO-NH-CO-R' | - | -imide | Succinimide | C4H5NO2 |
| Hydrazones | Hydrazone group | R-C=N-NH-R' | - | -hydrazone | Phenylhydrazine | C6H5NHNH2 |
| Azo compounds | Azo group | R-N=N-R' | Azo- | -azo | Azobenzene | C6H5N=NPh |
| Nitroso compounds | Nitroso group | R-NO | Nitroso- | -nitroso | Nitrosobenzene | C6H5NO |
| Isocyanates | Isocyanate group | R-N=C=O | Isocyanato- | -isocyanate | Methyl isocyanate | CH3NCO |
| Isocyanides | Isocyanide group | R-N≡C | - | -isocyanide | Phenyl isocyanide | C6H5NC |
| Pyrimidines | Pyrimidine group | C4H4N2 | - | -pyrimidine | Pyrimidine | C4H4N2 |
| Pyrazines | Pyrazine group | C4H4N2 | - | -pyrazine | Pyrazine | C4H4N2 |
| Pyridazines | Pyridazine group | C4H4N2 | - | -pyridazine | Pyridazine | C4H4N2 |
Sulfur Functional Groups
| Chemical Name |
Group |
Formula |
Prefix |
Suffix |
Example |
Structural Formula |
|---|---|---|---|---|---|---|
| Thiols | Sulfhydryl group | R-SH | Mercapto- | -thiol | Ethanethiol | CH3-CH2-SH |
| Sulfides | Thioether group | R-S-R' | Alkylthio- | -sulfide | Dimethyl sulfide | CH3-S-CH3 |
| Sulfoxides | Sulfinyl group | R-S(=O)-R' | Alkylsulfinyl- | -sulfoxide | Dimethyl sulfoxide | CH3-S(=O)-CH3 |
| Sulfones | Sulfonyl group | R-S(=O)2-R' | Alkylsulfonyl- | -sulfone | Diphenyl sulfone | C6H5-S(=O)2-C6H5 |
| Sulfonic acids | Sulfonic acid group | R-S(=O)2-OH | Alkanesulfonyl- | -sulfonic acid | Methanesulfonic acid | CH3-S(=O)2-OH |
| Sulfonyl halides | Sulfonyl halide group | R-S(=O)2-X | Alkanesulfonyl- | -sulfonyl halide | Methanesulfonyl chloride | CH3-S(=O)2-Cl |
| Thiocyanates | Thiocyanate group | R-S-C≡N | Alkylthiocyanato- | -thiocyanate | Ethyl thiocyanate | CH3CH2-S-C≡N |
| Sulfonic esters | Sulfonate group | R-S(=O)2-O-R' | Alkanesulfonyloxy- | -sulfonate | Methyl sulfonate | CH3-S(=O)2-O-CH3 |
| Thiosulfates | Thiosulfate group | R-S(=O)2-O-S-R' | Alkanethiosulfonyloxy- | -thiosulfate | Ethyl thiosulfate | CH3CH2-S(=O)2-O-CH2CH3 |
Phosphorus Functional Groups
| Chemical Name |
Group |
Formula |
Prefix |
Suffix |
Example |
Structural Formula |
|---|---|---|---|---|---|---|
| Phosphines | Phosphino group | R3P | Phosphino- | -phosphine | Tributylphosphine | (C4H9)3P |
| Phosphates | Phosphate group | RO4P | Alkyl/aryl- | -phosphate | Dimethyl phosphate | (CH3O)2PO |
| Phosphonates | Phosphonate group | RO3P | Alkyl/aryl- | -phosphonate | Dimethyl phosphonate | (CH3O)PO2 |
| Phosphoric esters | Phosphate ester group | RO3P | Alkyl/aryl- | -phosphate | Diethyl phosphate | (C2H5O)2PO |
| Phosphonothioates | Phosphonothioate group | RO2PSR' | Alkyl/aryl- | -phosphonothioate | Dimethyl phosphonothioate | (CH3O)PSCH3 |
| Phosphinic acids | Phosphinic acid group | R2PO2H | Alkyl/aryl- | -phosphinic acid | Dimethyl phosphinic acid | (CH3O)2POH |
| Phosphonodithioates | Phosphonodithioate group | RO2PS2R' | Alkyl/aryl- | -phosphonodithioate | Dimethyl phosphonodithioate | (CH3O)PS2CH3 |
| Phosphoric amides | Phosphoric amide group | RO2PNR'R'' | Alkyl/aryl- | -phosphoric amide | Dimethyl phosphoric amide | (CH3O)2P(NH2) |
Halogen Functional Groups
| Chemical Name |
Group |
Formula |
Prefix |
Suffix |
Example |
Structural Formula |
|---|---|---|---|---|---|---|
| Alkyl halides | Haloalkanes | R-X | None | -ane | Chloromethane | CH3-Cl |
| Alkene halides | Haloalkenes | R2C=CHX | None | -ene | 1,2-Dichloroethene | ClHC=CHCl |
| Alkyne halides | Haloalkynes | RC≡CX | None | -yne | 2-Bromo-1-pentyne | BrCH2CH2C≡CH |
| Aryl halides | Haloarenes | Ar-X | None | -aryl | Chlorobenzene | Cl-C6H5 |
| Acyl halides | Haloacyl compounds | R-CO-X | None | -oyl halide | Acetyl chloride | CH3CO-Cl |
| Alcohol halides | Haloalcohols | RO-X | None | -ol | 2-Chloroethanol | Cl-CH2CH2-OH |
Contents
Nucleic Acid
- Nucleic Acid StructureFunctions of NucleotidesAdding NucleotidesRNAmRNAtRNAtRNA and Amino acidsmicroRNARNA Cap StructureNucleic Acid TerminologyGenetics Material LocationsNucleosomeNucleoside Analogs
- Anabolism of Nucleic AcidsCatabolism of Nucleic AcidsNucleic Acids as Information StorageReplicationmRNA TranscriptionGenetic Code TableMutationRepairing MutationAptamersGene StructureTransposonsTransductionConjugation
Enzymes
- Enzyme NamingEnergy Is a Requirement for a Chemical ReactionEnzyme StructureVitamins and Their Coenzymatic FunctionsEnzyme Prosthetic GroupFactors Influencing Enzymatic ActivityEnzyme Inhibitors
- Feedback InhibitionLocation and Regularity of Enzyme ActionAnabolic and Catabolic ReactionsSynthesis and Hydrolysis ReactionsMultienzyme ComplexesEnzyme Summary
© 2015 nlightn. All-rights reserved.