Enzymes
Enzymes are proteins that act as catalysts in chemical reactions, meaning they speed up the rate of chemical reactions that occur in living organisms without being consumed or altered themselves.
Enzymes are essential for many biological processes, including metabolism, digestion, and cellular respiration. Enzymes are used in various applications, including food processing, industrial production of chemicals and pharmaceuticals, and medical diagnostics and therapies. Enzymes are also used as model systems to study the principles of protein structure and function, which has led to the development of new technologies and drugs.
Enzymes are proteins created from amino acid chains; they increase the rate of chemical reaction within living cells without themselves becoming permanently altered. They are biological catalysts and required for all living organisms.
Three or more amino acids are known as a polypeptide chain. Enzymes are macromolecules which range in size from a small polypeptide chain (approximately 100 amino acids) to large conglomerates of thousands of amino acids. One could say that enzymes are just really large proteins, but because enzymes are more complex with various utility they can no longer be placed in the protein category.
Enzyme Naming:
The naming structure of an enzyme includes the suffix -ase.
Enzymes are named according to the type of reaction they catalyze and the type of substrate they interact with. For example, proteases are enzymes that break down proteins, while amylases break down carbohydrates.
Enzyme names are typically composed of two parts: the suffix “-ase” and the prefix which discloses the substrate being acted upon or the type of reaction being catalyzed, or both. Most metabolic reactions require enzymes unique to their substrate - different enzymes have specific roles in reactions and cannot be “swapped out” to act on different substrates.
The naming system classifies the enzyme in one of six classes:
- Oxidoreductases transfer electrons from one substrate to another. (A subset of which is dehydrogenases, which transfer hydrogens from one compound to another.)
- Transferases transfer functional groups from one substrate to another.
- Hydrolases split or sever bonds in molecules with the addition of water.
- Lyases add groups to or remove groups from double-bonded substrates.
- Isomerases convert a molecule from one isomer to another.
- Ligases catalyze the formation of bonds with the input of ATP and the removal of water.
Enzyme Classification Based on Type of Chemical Reaction Catalyzed
Energy Is a Requirement for a Chemical Reaction:
All chemical reactions require energy. Enzymes can reduce amount of energy required to catalyze a chemical reaction.
Enzymes allow reactants to be converted into products faster. The products can be from a synthesis reaction, where a new substance is formed or a decomposition reaction, where a substance is broken down into its component parts.
Energy requirements of a chemical reaction:
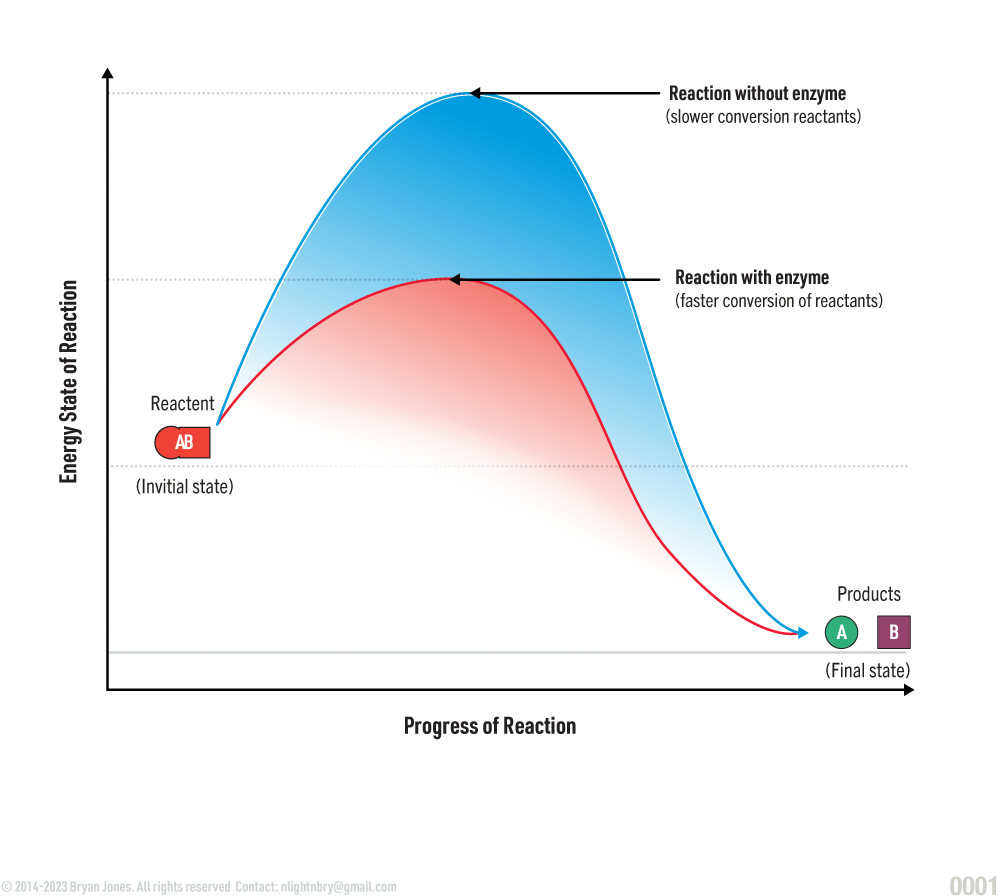
Enzyme Structure:
Enzymes have a specific structure which allows them to perform their role in a chemical reaction. The components of an enzymatic structure are as follows:
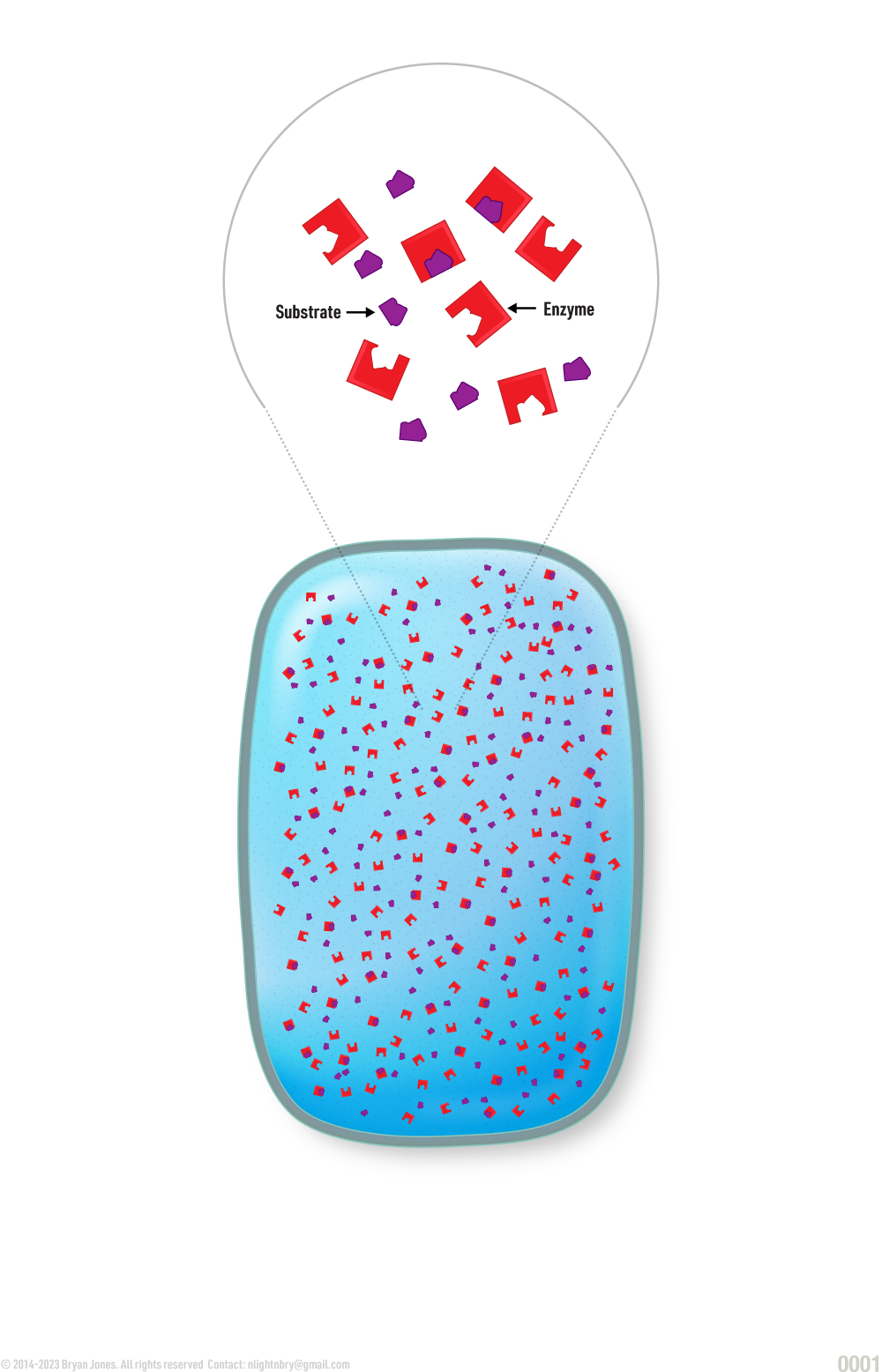
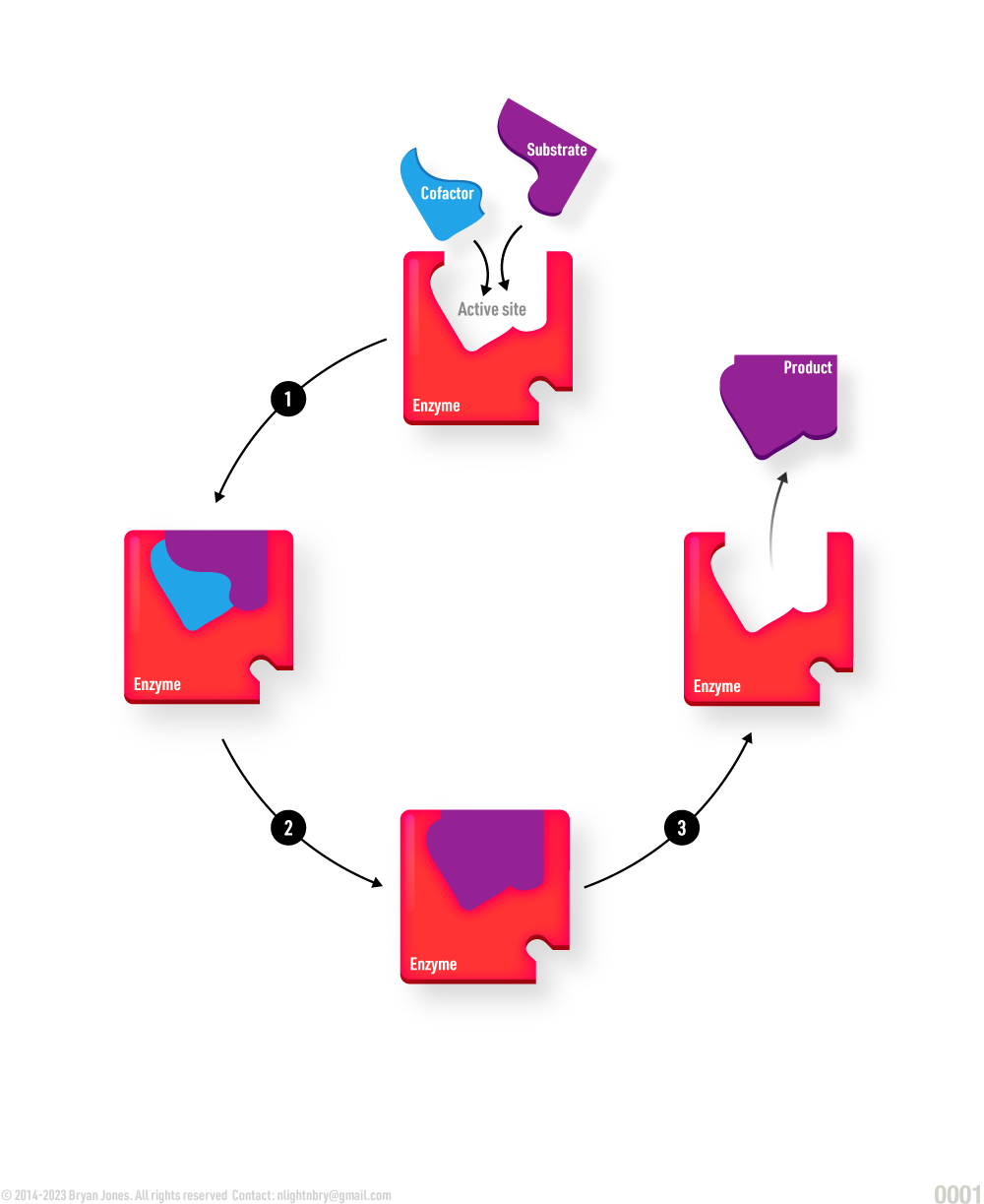
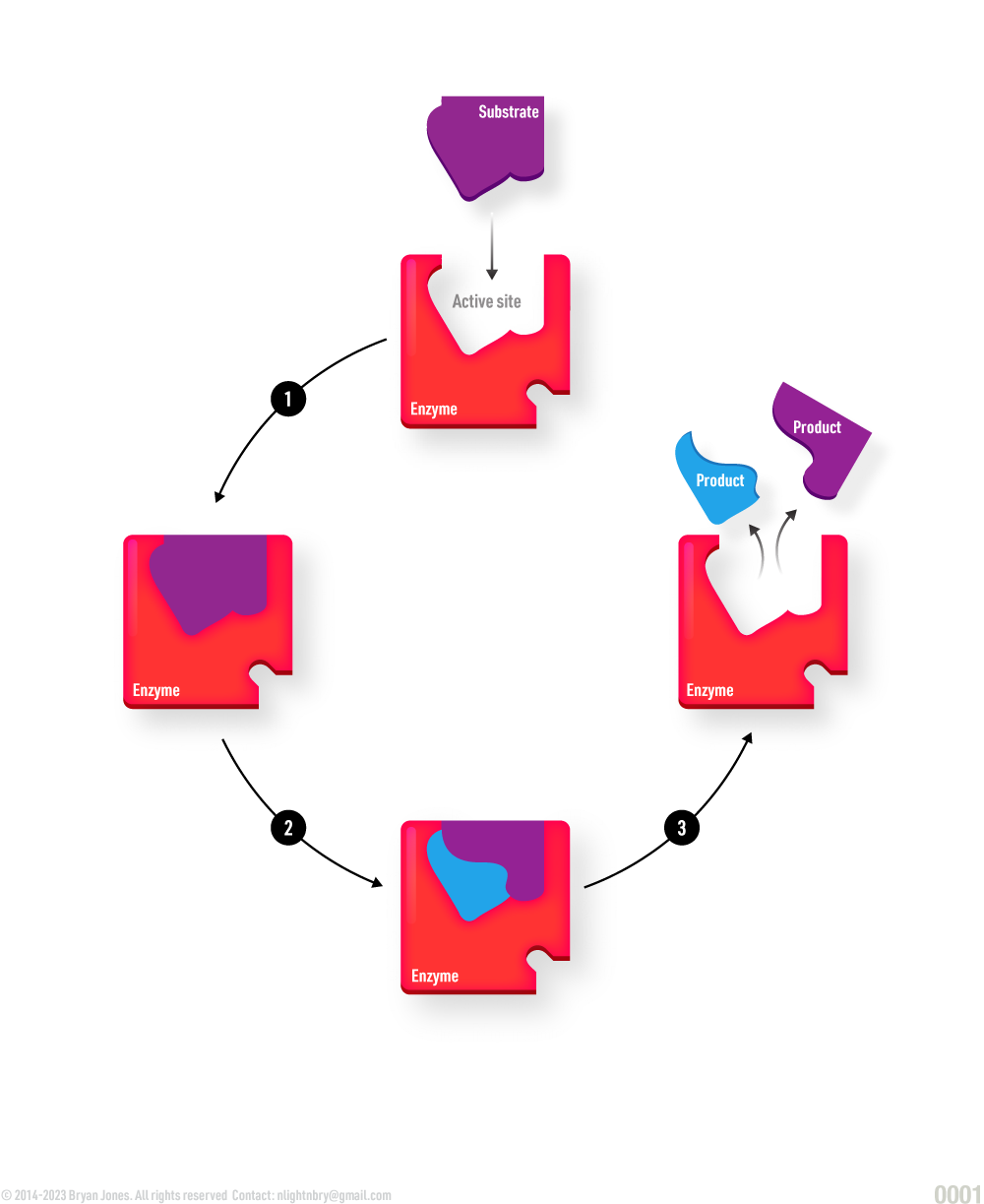
Enzymes have a specific three-dimensional structure that allows them to interact with other molecules called substrates, which they transform into products. The specific interaction between an enzyme and its substrate occurs at a region called the active site. The active site is a pocket or cleft in the enzyme that is lined with amino acid residues that are specifically arranged to fit the shape of the substrate molecule. The enzyme-substrate interaction is highly specific, meaning that each enzyme can only interact with a specific substrate or group of substrates that are chemically complementary to the active site. Enzymes can also be regulated by other molecules, such as inhibitors or activators, which bind to the enzyme and affect its activity.
- The active site is a crevice or groove in the enzyme where reactions can take place.
- The apoenzyme is the basic structure of the enzyme before it combines with other substances to create reactions.
- A cofactor is the component (nonprotein and commonly nonbiological) which fits like a lock and key into the active site of an apoenzyme. It helps “grab” molecules which the enzyme is to act upon.
- A coenzyme is a type of cofactor which is biological in nature.
- The substrate is the substance being acted upon by the enzyme.
- The holoenzyme is the complete enzyme, including the cofactor.
Apoenzyme Structure

Holoenzyme Structure

Enzyme-Substrate Complex Structure

Cofactors
Cofactors are small molecules that are required for the proper functioning of enzymes. They can either bind to the enzyme directly (inorganic cofactors) or bind to the enzyme and the substrate (organic cofactors).
Cofactors are considered non-organic. Some metallic cofactors include: iron, copper, manganese, zinc, cobalt, selenium. Metals activate enzymes, bring active site and substrate close together and participate directly in chemical reactions with the enzyme-substrate complex (Holoenzyme). Some cofactors function as electron acceptors and electron donors.
Inorganic cofactors include metal ions such as zinc, iron, magnesium, and copper. These ions can stabilize the enzyme-substrate complex or participate directly in the chemical reaction. For example, the enzyme carbonic anhydrase requires a zinc ion to catalyze the conversion of carbon dioxide to bicarbonate.

Coenzymes
Coenzymes are considered organic cofactors. Examples include: ATP and vitamins. The general function of coenzymes is to remove a chemical group from one substrate and add it to another. Coenzymes carry and transfer, electrons, hydrogen atoms, and Amino groups. Most important components of coenzymes are vitamins. Vitamins allow the formation of the (holoenzyme).
Organic cofactors or coenzymes are often referred to as coenzymes, and they are derived from vitamins or other small organic molecules. Coenzymes can participate in a variety of enzyme reactions by donating or accepting chemical groups such as electrons or functional groups. They can also act as carriers to shuttle small molecules between enzymes.
Other coenzymes include NAD+, which is derived from vitamin B3 and is involved in redox reactions, and coenzyme A, which is derived from pantothenic acid and is involved in the transfer of acetyl groups. Overall, cofactors and coenzymes are essential for enzyme function and allow enzymes to catalyze reactions at rates that are compatible with life.

Cofactors
Cofactors are small molecules that are required for the proper functioning of enzymes. They can either bind to the enzyme directly (inorganic cofactors) or bind to the enzyme and the substrate (organic cofactors).
Vitamins and Their Coenzymatic Functions:
As you may recall coenzymes are organic nonprotein molecules that bind with the protein molecule (apoenzyme) to form the active enzyme (holoenzyme).
Coenzymes are non-protein organic molecules that are required for enzymes to function properly. They act as helpers that assist enzymes in catalyzing reactions. Coenzymes are usually derived from vitamins and are essential for many metabolic pathways in the body. There are several categories of coenzymes, including:
- Nucleotide Coenzymes: These coenzymes are derived from nucleotides and include molecules such as ATP (adenosine triphosphate), GTP (guanosine triphosphate), and NAD+ (nicotinamide adenine dinucleotide). These coenzymes are involved in processes such as energy metabolism and signal transduction.
- Vitamin-derived Coenzymes: These coenzymes are derived from vitamins and include molecules such as coenzyme A (derived from vitamin B5), flavin adenine dinucleotide (FAD) and nicotinamide adenine dinucleotide phosphate (NADP+) (both derived from vitamin B2), and thiamine pyrophosphate (TPP) (derived from vitamin B1). These coenzymes are involved in processes such as carbohydrate metabolism, fatty acid synthesis, and the breakdown of amino acids.
- Iron-Sulfur Coenzymes: These coenzymes contain iron-sulfur clusters and are involved in electron transport reactions in the body. Examples include ferredoxin and cytochrome c.
- Heme-containing Coenzymes: These coenzymes contain heme groups and are involved in processes such as oxygen transport and oxidative metabolism. Examples include hemoglobin and cytochrome P450.
- Other Coenzymes: There are several other types of coenzymes that do not fit into the above categories. These include biotin, which is involved in carboxylation reactions, and lipoic acid, which is involved in oxidative decarboxylation reactions.
In summary, coenzymes are essential molecules that assist enzymes in catalyzing reactions in the body. They are derived from a variety of sources, including vitamins and minerals, and are involved in many metabolic processes in the body.
Selected vitamins and their function
Organic cofactors or coenzymes can be grouped into the following categories:
- Vitamins: These are organic molecules that are essential for the proper functioning of enzymes. Vitamins can be either water-soluble (e.g., B vitamins and vitamin C) or fat-soluble (e.g., vitamins A, D, E, and K).
- Coenzymes: These are non-protein organic molecules that are required for the activity of enzymes. Coenzymes include molecules such as NAD+, FAD, and coenzyme A, which participate in redox reactions and transfer chemical groups between molecules.
- Flavoproteins: These are proteins that contain a bound flavin cofactor, such as flavin mononucleotide (FMN) or flavin adenine dinucleotide (FAD), which are involved in redox reactions.
- Metalloproteins: These are proteins that contain a bound metal ion cofactor, such as iron, zinc, or copper. Metalloproteins are involved in a variety of biological processes, including oxygen transport, electron transfer, and catalysis.
- Thiamine pyrophosphate (TPP) and other prosthetic groups: These are non-amino acid components that are covalently attached to the protein portion of an enzyme and participate in catalysis. TPP is a cofactor for enzymes involved in carbohydrate metabolism.
- Pyridoxal phosphate (PLP) and other covalently attached cofactors: These are similar to prosthetic groups in that they are covalently attached to the protein portion of an enzyme and participate in catalysis. PLP is a cofactor for enzymes involved in amino acid metabolism.
- Coenzyme Q and other lipid-soluble cofactors: These are lipid-soluble molecules that act as electron carriers in the electron transport chain.
- Tetrahydrofolate (THF) and other one-carbon carriers: These are cofactors that are involved in the transfer of one-carbon units in metabolic reactions. THF is involved in the biosynthesis of purines and thymidine, among other processes.
Enzyme Prosthetic Group:
Enzyme prosthetic groups are non-protein molecules that are tightly bound to enzymes and are necessary for the enzyme's activity. These groups can either be covalently or non-covalently bound to the enzyme.
Covalently bound prosthetic groups are tightly bound to the enzyme through a covalent bond. These groups are often involved in catalysis or electron transfer. Examples include the heme group in cytochromes, which is involved in electron transfer, and the biotin group in carboxylases, which is involved in carbon dioxide transfer.
Non-covalently bound prosthetic groups are held in place by non-covalent interactions such as hydrogen bonds, electrostatic interactions, and van der Waals forces. Examples include metal ions such as zinc and magnesium, which are often involved in catalysis by stabilizing the transition state, and flavin adenine dinucleotide (FAD) and nicotinamide adenine dinucleotide (NAD+), which are involved in electron transfer.
Enzyme prosthetic groups are essential for the activity of many enzymes and are often involved in the enzyme's specificity and regulation. Their presence or absence can determine the overall activity of the enzyme and contribute to the diversity of enzymatic functions in living organisms.
Factors Influencing Enzymatic Activity:
Enzymes are controlled by the cell environment, allowing them to function at different rates of activity. The number of enzymes present can have an effect on their ability to control chemical reactions. Also, the enzymatic activity can be controlled by inhibitors. Environmental factors can influence the activity of an enzyme as well. These include temperature, pH, and substrate concentration.
Temperature:
Enzymes function best at ideal temperatures. Too cold, and their ability to increase chemical reactions is hampered. Too hot and the shape of the enzyme itself can change, resulting in their inability to bond with its substrate.
Optimal Temperature

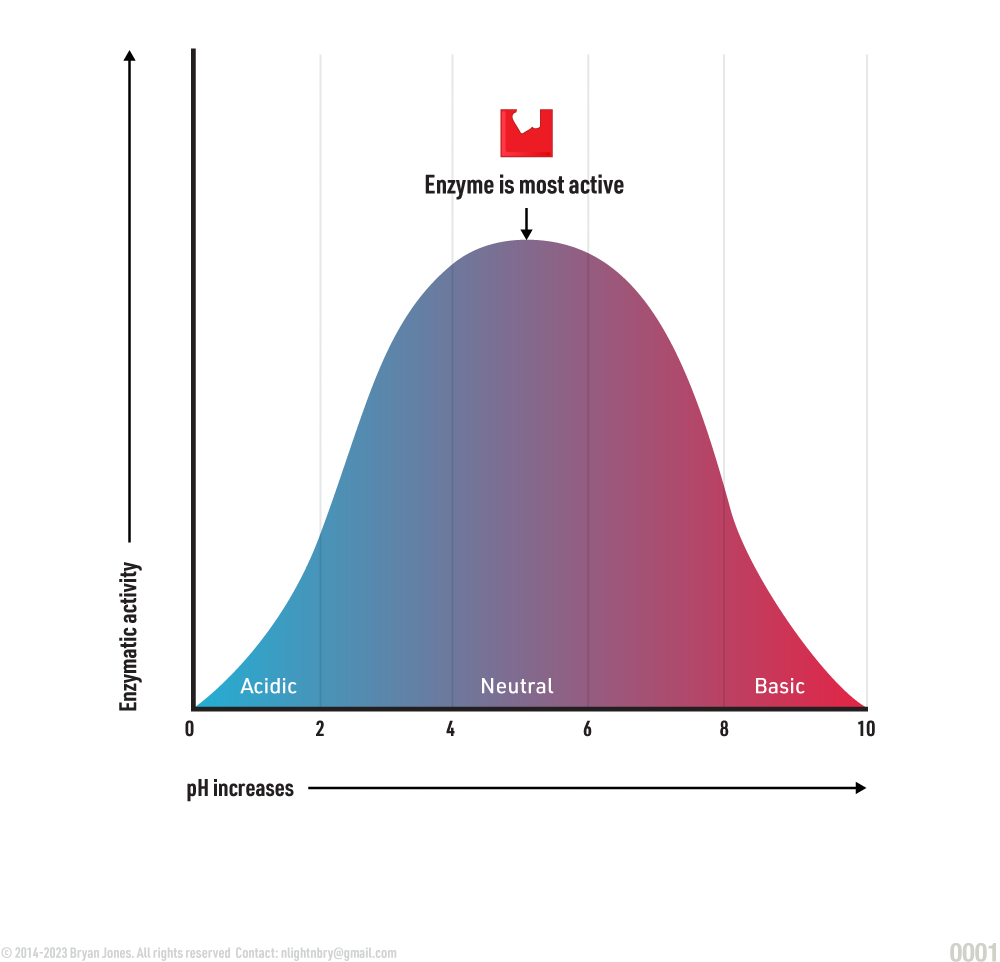
pH:
Enzymes function best at a specific pH value. When the pH balance is off, the enzyme’s ability to react is hampered. If the concentration of H+ is changed too drastically, a protein’s structure is altered and therefore cannot “connect” with specific enzymes anymore. When such conditions become extreme, a protein structure can unfold and result in denaturation.
Optimal pH
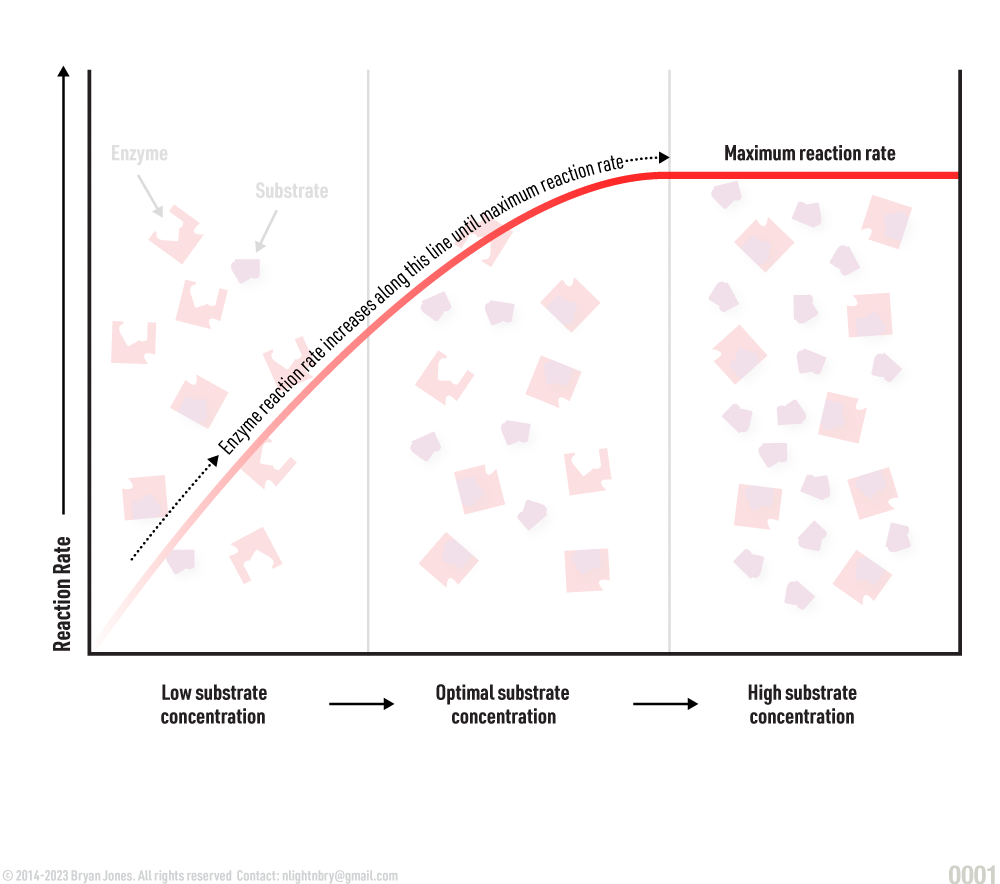
Substrate concentration:
The rate of reaction increases with the number of substrate molecules. Once all the enzyme molecules’ active sites are filled, they can no longer perform their function. In short, the enzymes are “used up.”
Substrate Concentration

Enzyme Inhibitors:
Enzyme inhibitors are competitive or noncompetitive, inhibitors exist to control rates of chemical reactions, and specifically, cell growth which will later be referred to as metabolism.
Competitive Inhibitors:
A competitive inhibitor prevents an enzyme from its function by entering the active site and preventing the lock-and-key fit with the substrate.
Non-competitive, or Allosteric Inhibition:
The Allosteric site of an enzyme is another lock-and-key site of the enzyme. Instead of being the location where a substrate can have its reaction, the Allosteric site affects the shape of the enzyme, thus influencing its ability to interact with substrates. Binding at this site causes an enzyme to become nonfunctional.
An uninhibited enzyme and its substrate:

Competitive inhibitor blocks the enzyme's active site:

Non-competitive inhibitor affects the enzyme's active site shape:

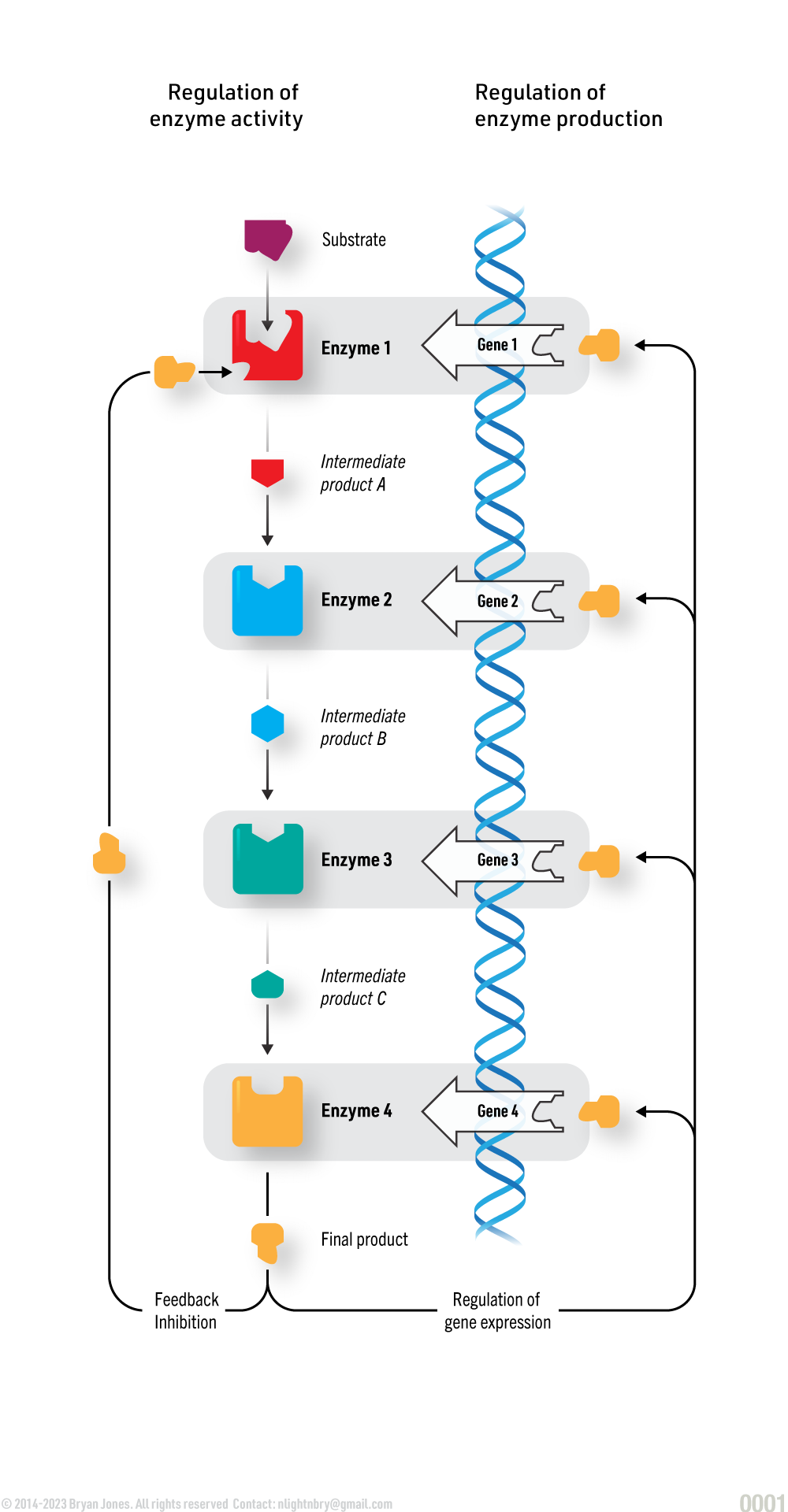
Feedback Inhibition:
Enzymes have control mechanisms which can shut them down.
Another form of Allosteric inhibition is feedback inhibition, or end-product inhibition, which stops a cell from wasting chemical resources by making more of a substance than needed. In the graphic below you will see that enzymes produce a product that will eventually shut down the metabolic pathway.
Feedback Inhibition:

Allosteric Feedback

Another way to visualize feedback inhibition:
Location and Regularity of Enzyme Action:
Enzymes can act internally or externally of a cell.
Enzymes are produced within a cell, though they can exist outside of their cell of origin. They can perform their functions inside or outside of a cell. Exoenzymes can break down (hydrolyze) large food molecules or harmful chemicals outside of a cell. Endoenzymes function within a cell. While cells are where enzymes are created, they are not produced at the same rate or in equal amounts. Within a cell, constitutive enzymes exist regardless of the quantity of substrate and are always present and in nearly constant quantities. The concentration of regulated enzymes in a cell increases or decreases in response to substrate levels. Enzyme production is either turned on (induced) or turned off (repressed) based on substrate concentration.
Endoenzymes
Exoenzymes

Anabolic and Catabolic Reactions
Anabolic and Catabolic reactions are basic mechanisms in which organisms build & breakdown matter.
Anabolic Reaction
Catabolic Reaction
Synthesis and Hydrolysis Reactions:
One type of synthesis reaction is known as dehydration reaction. For each bond made, they release one water molecule, and require ATP for the energy to do so. Anabolism, a synthesis reaction, requires enzymes and smaller substrate molecules for the formation of covalent bonds. In catabolism, reaction require energy transactions. This results in reshaping of the cellular structure.
Dehydration Reaction (anabolic):
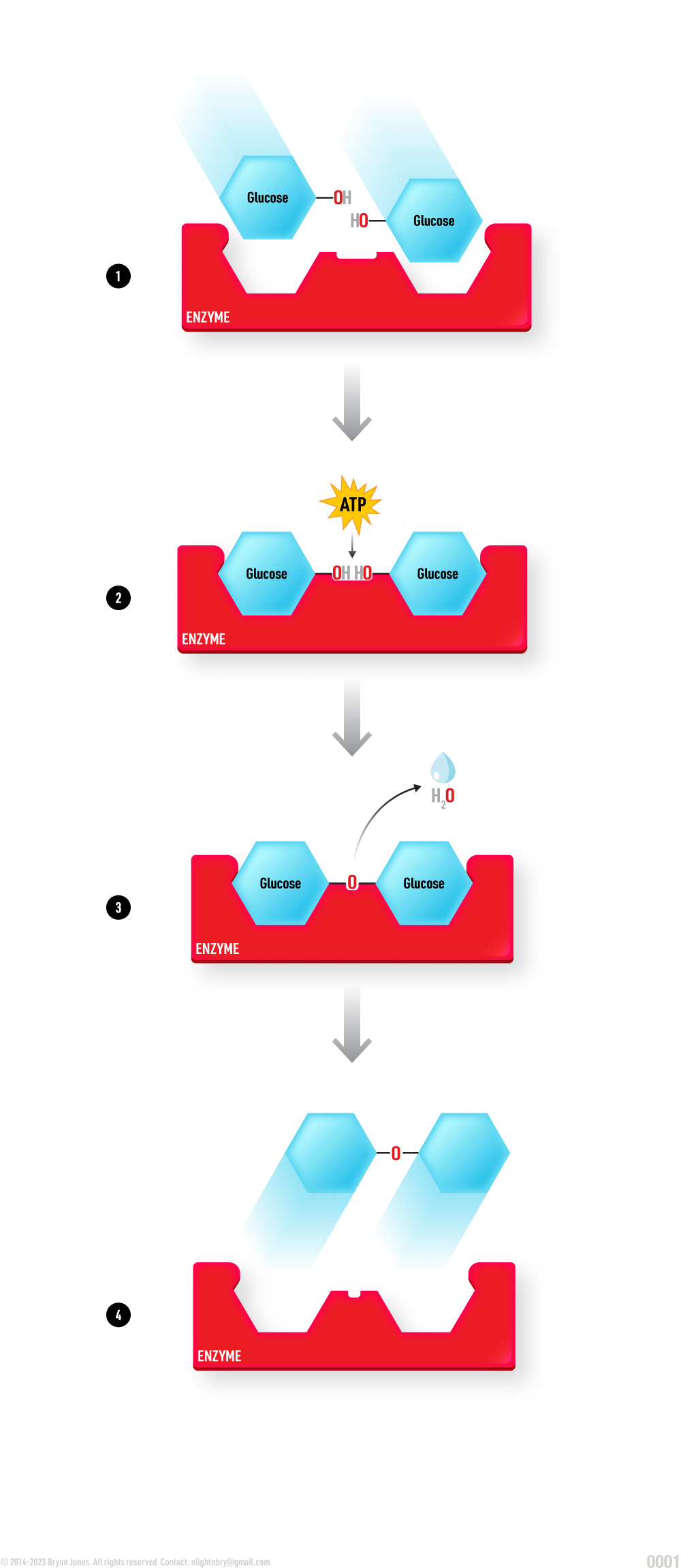

Two Glucose molecules are attracted to the Enzyme's active site due the electromagnetic force between opposing charges.

Once the glucose fit into the grooves of the Enzyme's active site, a catalytic reaction begins. ATP from the surrounding solution provides the energy to bonds these two glucose molecules together.

A reaction has occurred, H+ is cleaved from Glucose 1 and a OH- group is cleaved from Glucose 2. H2O is spontaneously formed due proximity and the electromagnetic force between opposing charges. The ATP is utilized and becomes ADP.

The newly formed compound maltose detaches from the Enzymes because it no longer has the same charge and joins the surrounding solution.
Hydrolysis Reaction (catabolic):
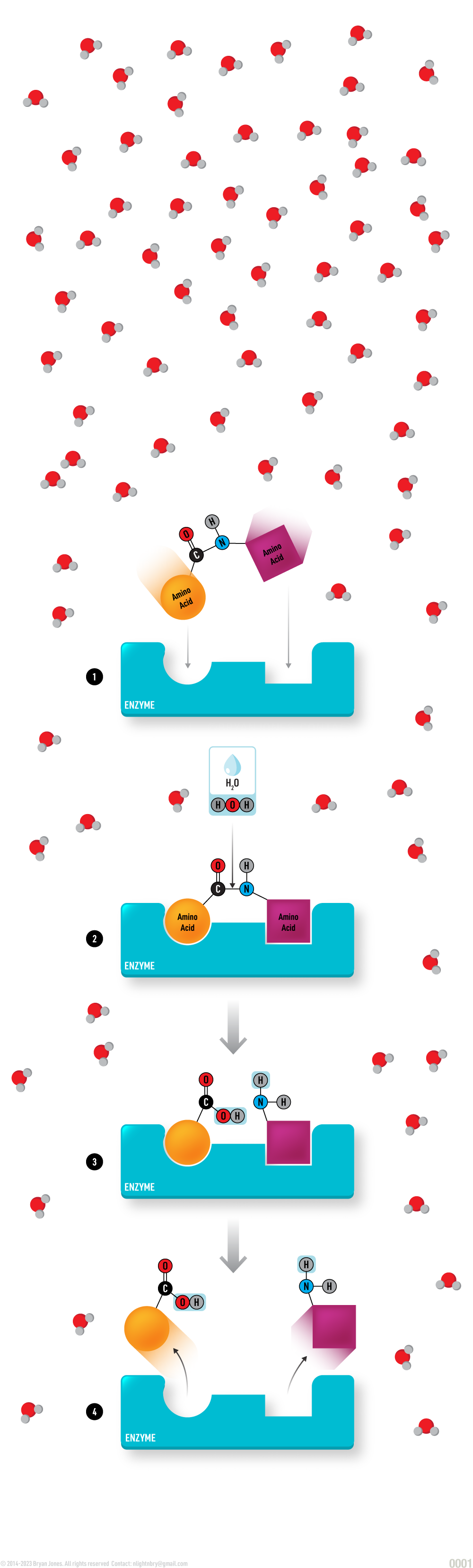

A compound in aqueous solution consisting of two amino acids is attracted to a nearby holoenzyme due to electromagnetic force between opposing charges.

A compound consisting of two amino acids comes in contact with an holoenzyme in aqueous solution.

The substrate consisting of two amino acids undergoes a catalytic reaction due to water and the enzyme's cofactor/coenzyme.

A water molecule cleaves the bond between a carbon-nitrogen bond forming two separated amino acids. Amino acid 1 gains an OH group forming a carboxyl functional group, while amino acid 2 accepts Hydrogen completing the amino functional group.
Multienzyme Complexes
Multienzyme complexes are groups of enzymes that work together to perform a specific metabolic function. These complexes are often associated with metabolic pathways that require multiple enzymatic steps. By bringing the enzymes together, multienzyme complexes can increase the efficiency of the pathway, by reducing the loss of intermediates and the diffusion of substrates between enzymes.
Multienzyme complexes can be found in a variety of biological systems, including prokaryotes and eukaryotes. Some well-known examples of multienzyme complexes include the pyruvate dehydrogenase complex involved in the conversion of pyruvate to acetyl-CoA, the fatty acid synthase complex involved in the synthesis of fatty acids, and the ribosome involved in protein synthesis. In general, the formation of multienzyme complexes is regulated by several factors, including the presence of specific protein domains, post-translational modifications, and protein-protein interactions.
Enzyme Summary
Enzymes are biological catalysts that accelerate the rate of chemical reactions in living organisms without themselves being consumed or changed. Enzymes play a critical role in nearly all biological processes, including metabolism, signal transduction, and DNA replication and repair. The in-depth contents of enzymes can be broadly classified into the following:
Overall, the in-depth study of enzymes is critical to understanding the complex biochemical processes that occur in living organisms and has significant implications for medicine, biotechnology, and other fields.


