ATP: Adenosine Triphosphate
ATP transports chemical energy (energy held between bonds) within cells to build (anabolic) and disassemble (catabolic) compounds.
ATP has unstable, “high energy” bonds. The key characteristic of these bonds is not their strength but their ability to release energy quickly. When these ATP bonds break they releases large amounts of energy quickly
When energy is spread throughout a nutrient molecule, it is difficult for the cell to use. Therefore a cell utilizes enzymes powered by previous reactions to break down nutrients while synthesizing ATP. When energy is stored within ATP, it becomes more useful to a cell and energy can be spent.
Energy in Chemical Reactions:
All chemical bonds require energy to be formed or broken. The release of chemical energy occurs when bonds are formed.
A bond is formed releasing energy

When a bond breaks it releases energy

Structure of ATP:
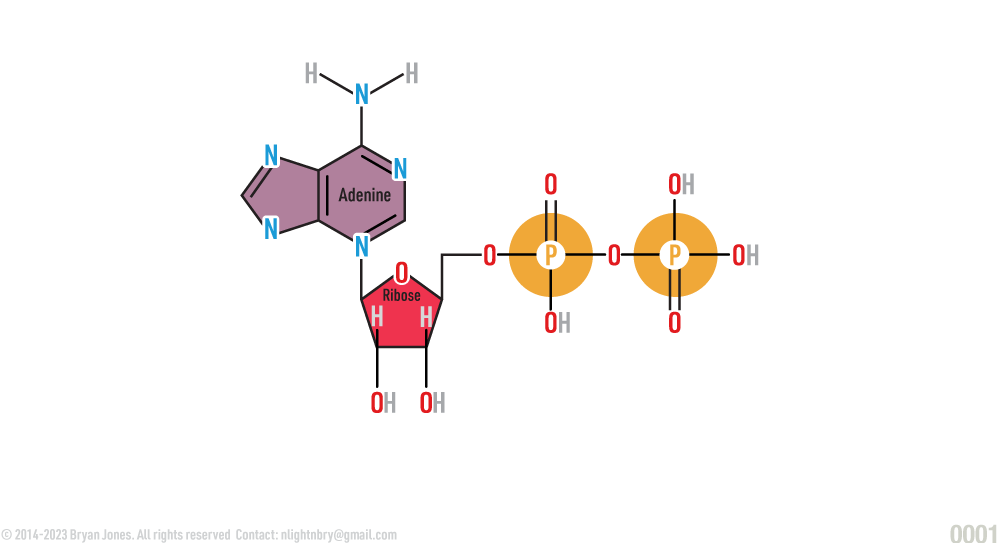
ATP is a nucleotide containing adenine, ribose, and three phosphate groups.
ATP is a three-part molecule consisting of adenine (a nitrogenous base) linked to a ribose (5-carbon sugar), with a chain of three phosphate groups bonded to the ribose. The phosphate groups in ATP carry negative charges. The last two phosphate groups of the three in ATP are strained by their negativity and proximity. Due to entropy of a system, molecules invariably reach a lower energy state.
Adenosine Triphosphate (ATP)

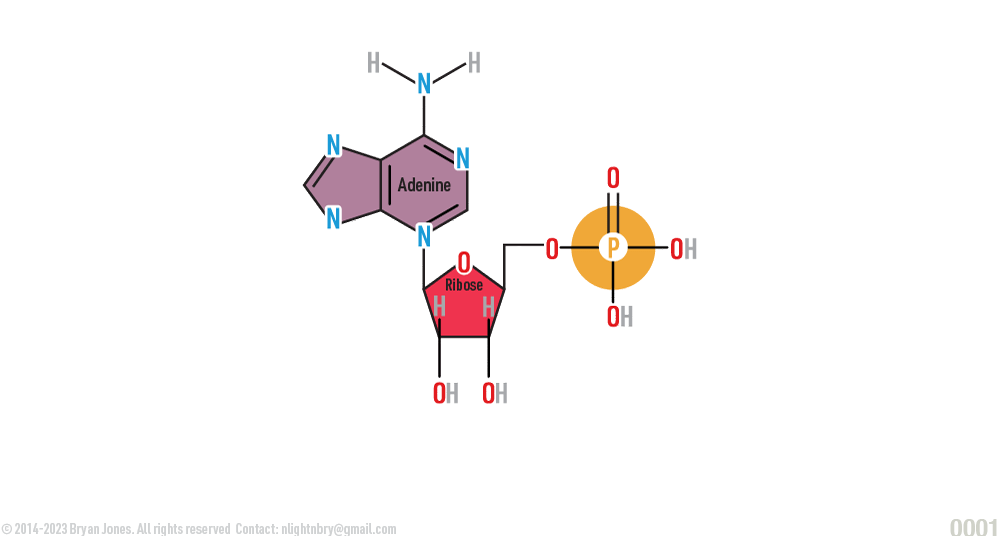
Adenosine Diphosphate (ADP)
Adenosine Monophosphate (AMP)
Reaction Process
How ATP is made
ATP is formed when ADP and energy create a exergonic reaction. Exergonic reactions are chemical reactions which release more energy than required for bond formation. The reaction occurs along the lipid membrane of the cell's mitochondria with the help of ATP synthase. The difference between ATP and ADP is the number of phosphate groups. AMP has one phosphate group.
The difference between ADP and ATP

ATP Hydrolysis
When ATP is hydrolyzed it releases energy and an inorganic phosphate.
The reaction process of forming ADP from ATP is catalyzed by hydrolase which catalyze the hydrolysis of a chemical bond. The word hydrolysis comes from the roots hydro (water), and lysis (loosen).
When a water molecule is added to ATP, chemical energy in ATP is released, along with an inorganic phosphate group, leaving a remainder of ADP. This occurs often and easily in muscles, where energy is needed to do work.
Hydrolysis Reaction:
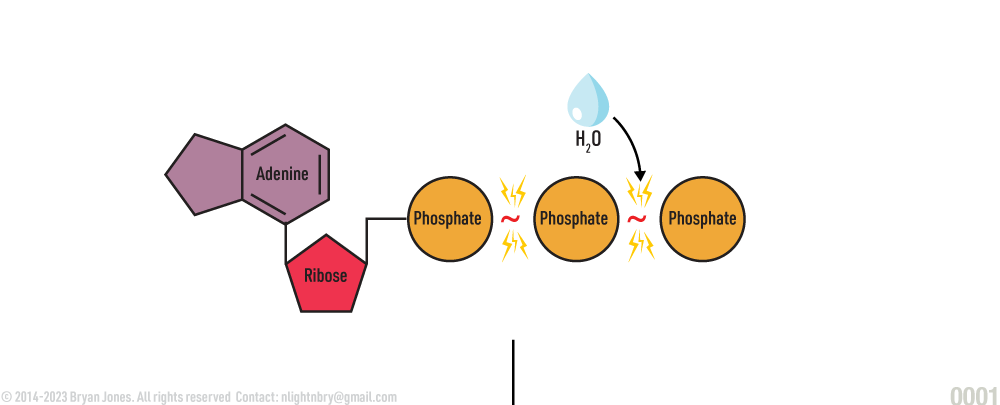
Water hydrolyzes the bond between the second and third phosphate splitting off one phosphate used to fuel other reactions
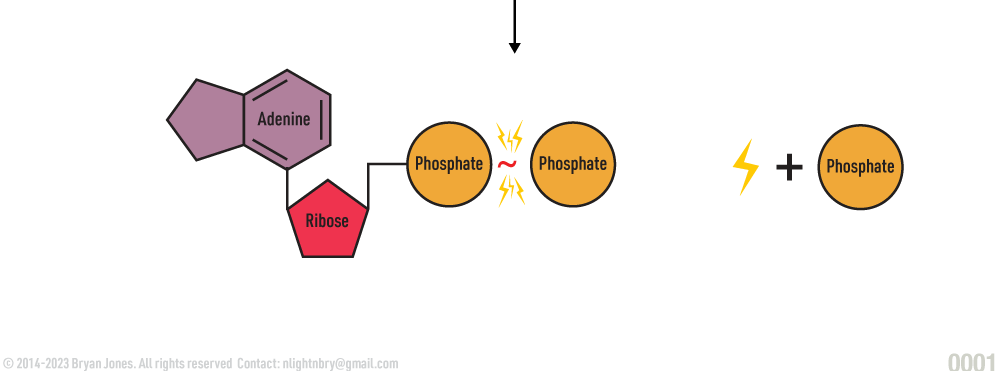

ADP and a phosphate group are ready to be combined.

Exergonic reaction yields energy which is used to form ATP.

A molecule of ATP is ready for use.

ATP is used in endergonic reactions.
ATP in Coupling Anabolic and Catabolic Reaction:
Bonds broken & formed release energy
When energy is involved in the splitting or combining of molecules using ATP, heat is always a byproduct.
If the molecules are being split apart (catabolism), the remaining energy is stored in ATP. ATP is used to combine complex molecules (anabolism), transport substances across membranes, and motion produced by muscle contraction. ATP use and replenishment is an ongoing cycle.
It takes ATP to make ATP within a cell, when ATP is produced It releases some energy which continues the production cycle of ATP. Removal of the terminal (third) phosphate group releases free energy ΔG = -30.5 kJ/mol (-7.3 kcal/mol). One glucose produces 38 ATP x 31 kJ/mol = 1178 kJ/mol of energy for a cell to utilize.
- ADP and a phosphate group are ready to be combined.
- Exergonic reaction yields energy which is used to form ATP.
- A molecule of ATP is ready for use.
- ATP is used in endergonic reactions.
ATP Generation Cycle

| Cellular Process | Energy Required (in ATP) |
|---|---|
| Glycolysis | 2 ATP (61 kJ) |
| Krebs Cycle | 2 ATP (61 kJ) |
| Electron Transport Chain | 34 ATP (1037 kJ) |
| Active Transport | Varies, but can be up to 30 ATP per molecule transported (915 kJ) |
| Muscle Contraction | 4 ATP per contraction (102 kJ) |
| Protein Synthesis | Varies, but can be up to 20 ATP per amino acid added (610 kJ) |
| Cell Signaling | Varies, but can be up to 10 ATP per signal molecule activated (305 kJ) |
| DNA Replication | Varies, but can be up to 100 ATP per DNA base pair replicated (3050 kJ) |
| Cell Growth and Division | Varies, but can be up to 1000 ATP per cell division (30500 kJ) |
How to better understand energy
- Electrical Appliances: The energy consumed by electrical appliances in your home is often measured in joules. For example, if a 100-watt light bulb is turned on for 10 seconds, the energy consumed is 100 watts * 10 seconds = 1000 joules.
- Chemical Reactions: When your body metabolizes food, it converts the energy stored in the food into various forms, including mechanical energy for movement and heat. The energy released during a chemical reaction can be measured in joules. For instance, the combustion of 1 gram of carbohydrates releases about 17 kilojoules (17,000 joules) of energy.
- Photons: As mentioned earlier, the energy of a photon of light is measured in joules. For instance, a photon of blue light with a wavelength of about 475 nanometers carries an energy of approximately 4.15 x 10^-19 joules.
Lifting an Object: If you lift a 1-kilogram book vertically by 1 meter, you are doing mass against gravity. The amount of energy you've expended is equal to the weight of the book. In the case of lifting an object, the force is the weight of the object, which is equal to its mass times the gravitational acceleration. So, the equation becomes about calculating gravitational potential energy:
Energy = Mass * Force * Distance
Potential Energy (U) = Mass (m) * Gravitational Acceleration (g) * Height (h)
- Potential Energy (U) is the energy an object possesses due to its position in a gravitational field.
- Mass (m) is the mass of the object.
- Gravitational Acceleration (g) is the acceleration due to gravity in the location where the object is situated.
- Height (h) is the vertical distance between the object and a reference point (usually the ground).
The potential energy calculated using this formula will give you the amount of energy required to lift the object to the specified height from its initial position. Keep in mind that this calculation assumes there's no energy loss due to factors like friction, air resistance, or other dissipative forces.
For example, if you lift a 1-kilogram book vertically by 1 meter, the energy expended is equal to:
Energy = 1 kilogram * 9.81 m/s2 * 1 meter
Energy = 9.81 joules
As you can see, the energy expended is equal to the weight of the object multiplied by the distance lifted. The greater the mass of the object or the distance lifted, the greater the amount of energy expended.
The formula for calculating potential energy is not equal to work or energy. Work and energy are related concepts, but they have different formulas. Work is given by:
Work (W) = Force (F) * Distance (d) * cos(θ)
- Work (W) is the amount of energy transferred when a force is applied over a distance.
- Force (F) is the force applied to the object.
- Distance (d) is the distance over which the force is applied.
- θ is the angle between the direction of the force and the direction of displacement.
Energy, in general, is a broader concept and can come in various forms, such as potential energy, kinetic energy, thermal energy, and more. The relationships between these different forms of energy involve different formulas and concepts.
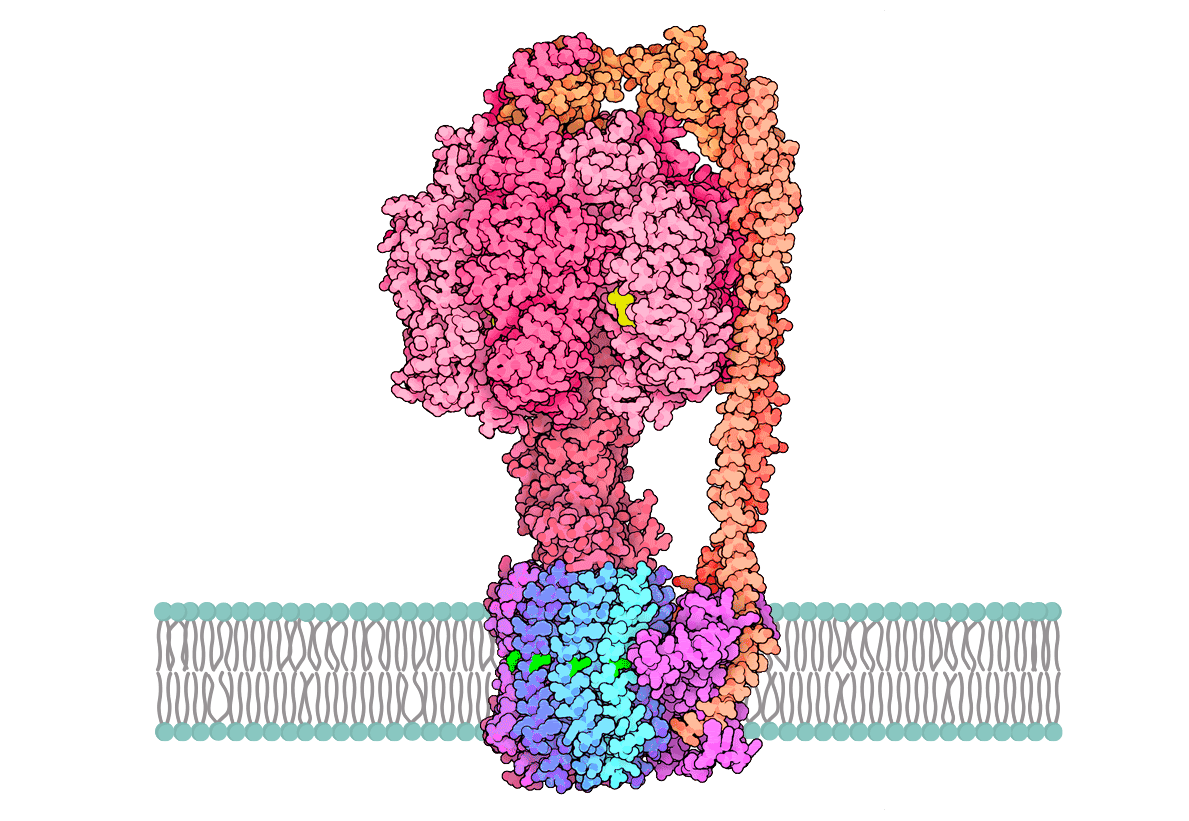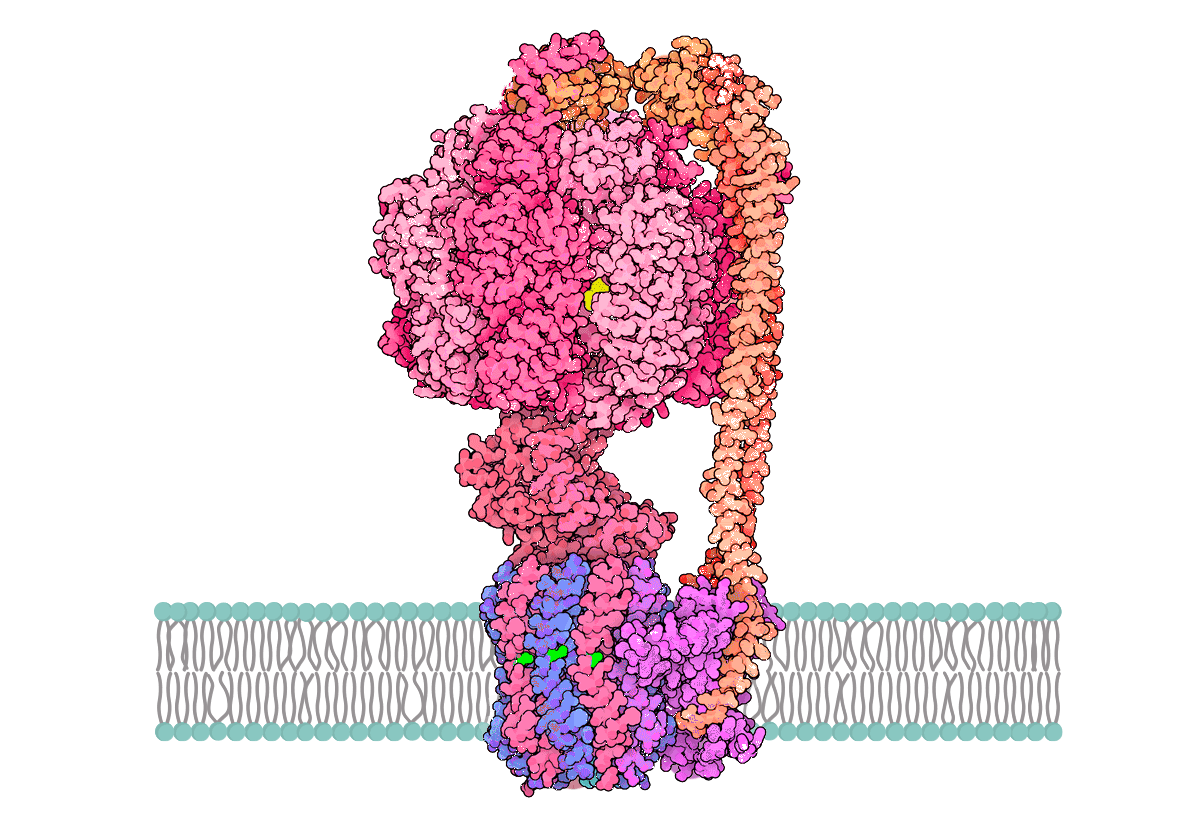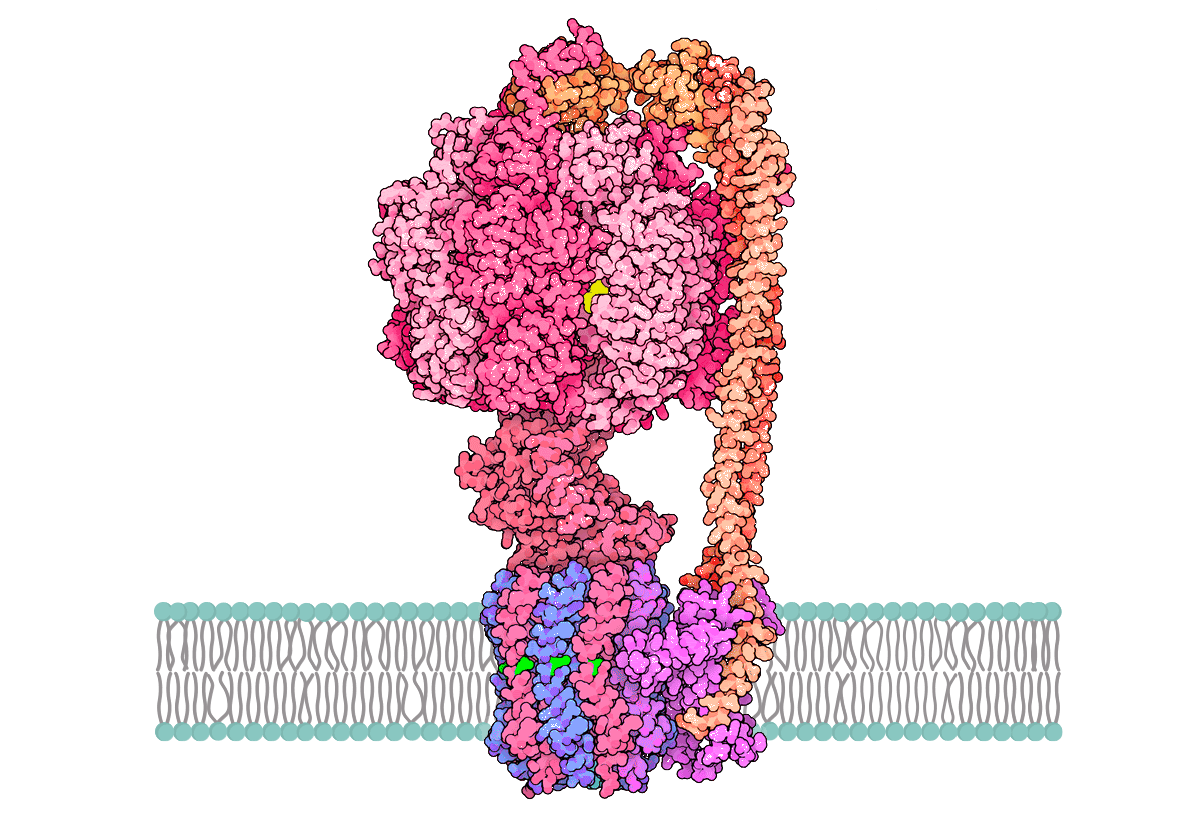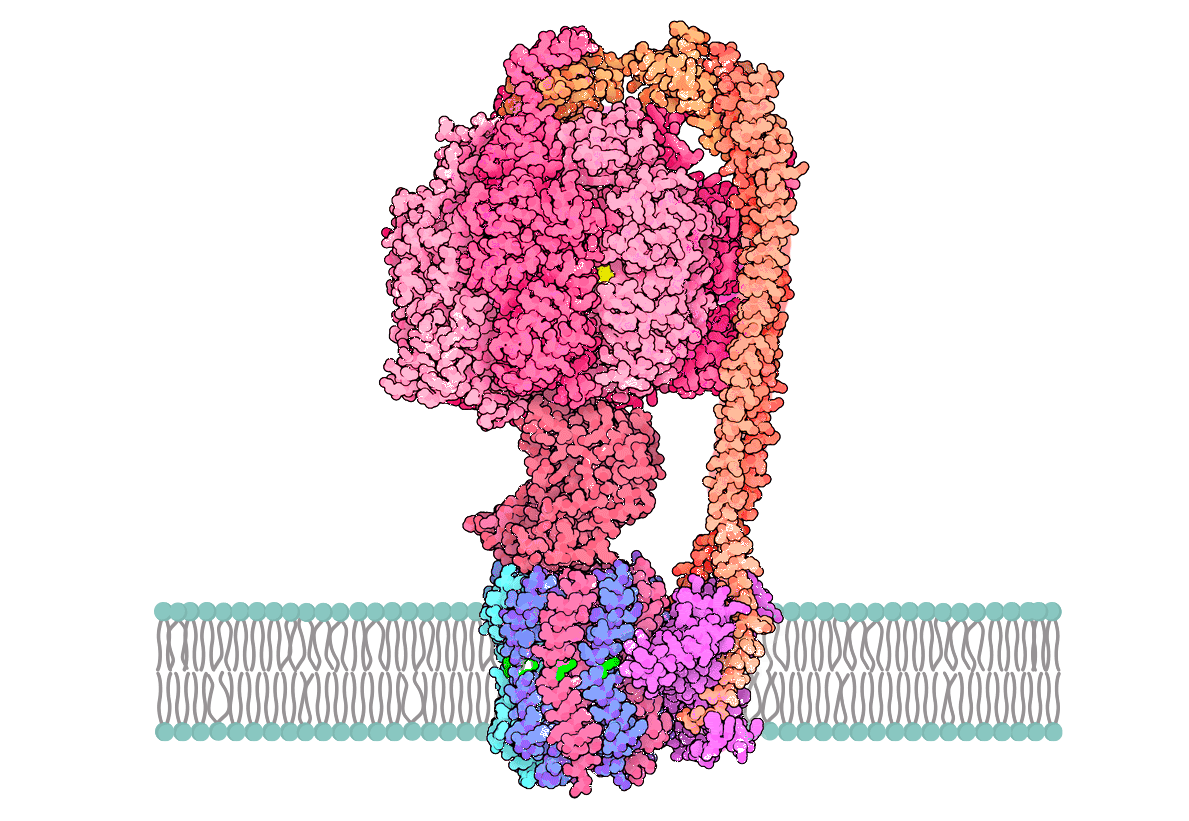
ATP Synthase and ATP Production
John Walker shared his Nobel Prize with Paul Boyer for uncovering ATP synthesis. Here's an animation explaining ATP synthase, which harnesses proton motive force for ATP production. This enzyme works within organelle membranes, converting ADP and phosphate into ATP through a rotary mechanism. The central rotor rotates about 150 times per second. Our bodies generate ATP equal to our weight daily, sustaining our lives.
ATP Synthase