Solutions
The components in solutions are called solutes and solvents, or dissolving mediums. The essential characteristic of a solution is the uniform dispersal of solutes in the dissolving medium.
Several common types of solutes are acids, bases and salts. The strength of a solution containing acid or a base is called the pH. When these solutes are added to the solvent water, an exchange of ions occurs.
Solvent and Solute
A solvent separates atoms, and those separated atoms are the solute.
A mixture is a material composed of two or more substances. The major component of a solution, called the solvent, is typically the same phase as the solution itself. Each minor component of a solution (and there may be more than one) is called the solute.
Solutions come in all states (solid, liquid, gas), and the solvent and the solute do not have to be in the same state to form a solution (such as salt and water).
The solutes are the two individuals in the relationship which interact with the solvent. When the couple gets wet (Aq) it splits, now you're thinking, "being wet is usually a good thing". Well the couple split and each solute is wet with a H2O couple, H2O dissolved them. Think of a molecular couple submerged in an aqueous solution the couple separate because someone got in between those bonds. Someone sprayed the molecular couple with water.
Heterogenous - solutions are those that are unevenly mixed.
- Salad dressing (oil and vinegar)
- Orange juice with pulp
- Sand in water
- Italian dressing (oil, vinegar, herbs, and spices)
- Muddy water
Homogenous - solutions are those that are evenly mixed.
- Saltwater
- Sugar dissolved in water
- Air (a mixture of gases)
- Lemonade (sugar and lemon juice dissolved in water)
- Vinegar
Ionization
In ionization, molecules break apart into ions, either cations and anions.
Key characteristics of functional groups include: having consistent chemical properties and their atoms are linked to by covalent bonds. They behave as special accessory molecules which bind to organic compounds. The arrangement of a functional group makes it possible to predict how a new compound will react.
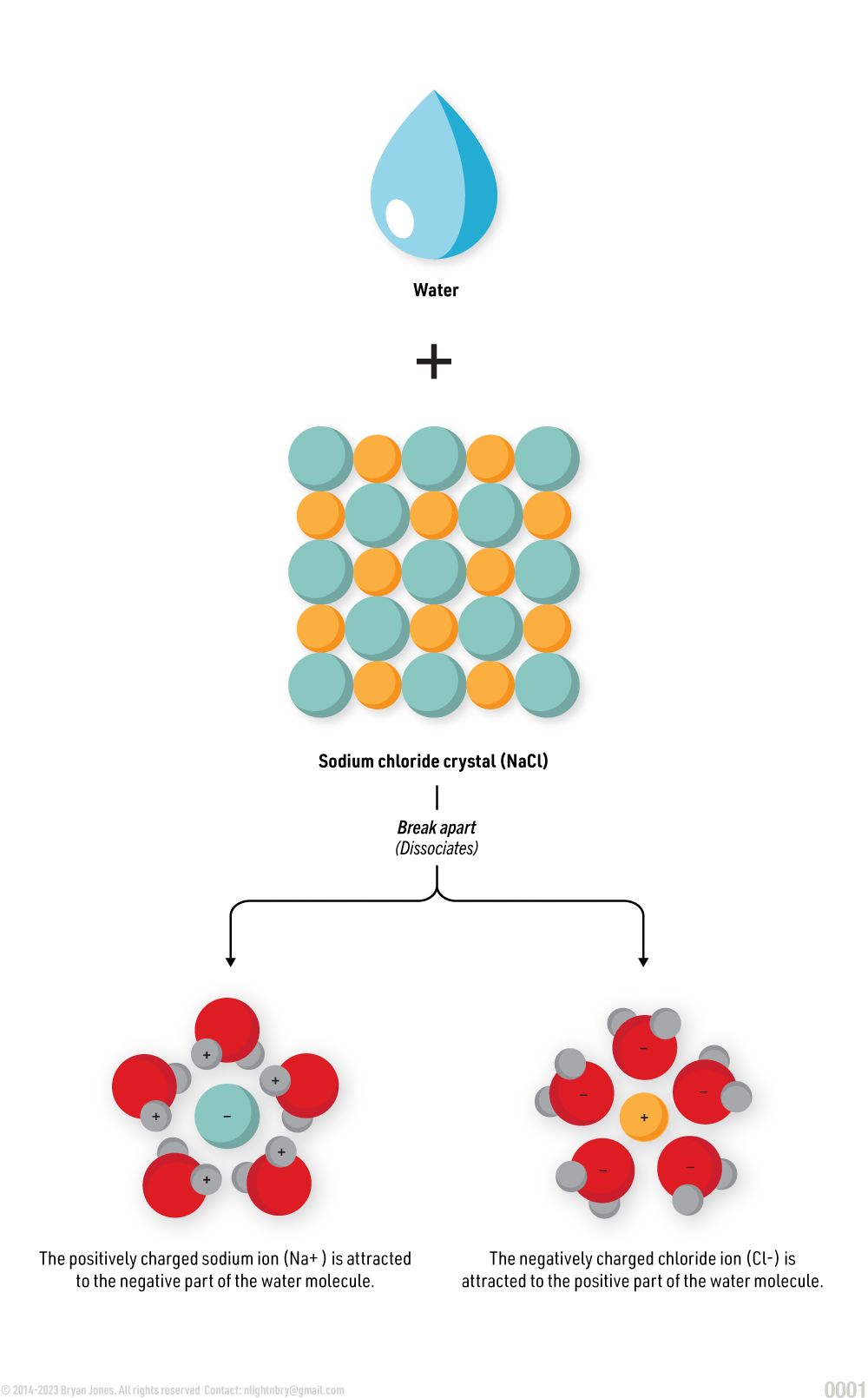
When dissolving mediums, such as inorganic salts, are dissolved in a solute, such as water, they undergo ionization (the dissolving medium breaks apart into ions).
Even a glance can ionize your relationship. Laser beams aren't the only thing causing separation. The influx in electrical charge tends to change circumstances.
(+) Don't be jealous, that's not helping. We're two grown adults, it was just a glance. I was just getting some sun, if I want shade I'll ask you how you feel about it.
(x) Just a glance? You're pheromones and hormones up-regulated.
(+) Yeah ... well I'm not the only one.
(x) Two grown adults?
(+) Yes.
Meanwhile, back at the crystal sanctuary, Eye beams is drilling holes, "some pulsed pumping guarantees further oscillation".
(+) Lidar, that's a good attribute, what can you do with that?
(–) Measuring your curve enables me to transition between isometric states.
(+) I guess that explains why it's getting hot in here.
(–) Yes, I took my glasses off, this helps.
(+) 🤔, I disagree.
(–) I wonder what the spectroscopy measurement would be.
(+) I don't.
(–) I bet it looks like Venus. I'll open a window.
(+) I meant the one made of glass.
Note the (-) indicates an electron (+) represents a proton, both of which have opposite charges which attract each other. Like charges, (+)(+) repeal each other until you put a (-) between, ex. (+) | (+) . Now a – usually indicates a bond between two elements, but technically that’s a em dash and the symbol used for electrons is a superscript minus- which is different. Similar but different. We haven't got to chemical Shorthand yet, but it will come in hand to know so lick here–

In the presence of water, the bonds between the Na+ and Cl- are disrupted, and the NaCl dissolves in water.
Ionization Process:
Hydronium and Hydroxide Formation
When two water molecules are hydrogen bonded, sometimes the hydrogen involved will shift from one water molecule to the other. The result is a positively charged hydronium ion (H3O+) and a negatively charged hydroxide ion (OH-).
The ion H3O, consisting of a protonated water molecule and present in all aqueous acids. An H+ and an OH- ion from a single water molecule, although in real life a free H+ ion would always attach itself to an available water molecule rather than float freely. In pure water this only happens occasionally by chance and nearly all the water molecules have no charge H2O.
Last time on the Network, we were watching two swinger couples interact with other locals. Looks like one couple of H2O split and formed their own Hydronium H3O+ and the remaining is now called a HO- group (Hydroxide).
Hydronium and Hydroxide Formation:

-HO vs OH-
There is a difference between -HO and OH-. The notation (-OH" represents a hydroxyl group (-OH) attached to an organic compound, typically denoting an alcohol functional group. The hydroxyl group (-OH) consists of an oxygen atom bonded to a hydrogen atom and is characteristic of alcohols.
HOs come in different sizes. It's best to know what kind of HO you're associating with. A -HO is by herself, I mean she has a little hydrogen friend tagging along with her electron. If you remain positive you should meet-up. As an electron (-) you're looking for -OH. You have to watch out for the HO- they're part of a neutral group. These groups can bond with a promiscuous carbon atom containing other holes to fill, carbon readily excepts electrons. Carbon requires 4 additional electrons to complete its octet (8 valence electrons)
If you're looking for a spark with Oxygen who is part of your local hydroxide, you could give her banana, or be a little salty in person, "Her and I will make a good pair" – Sodium hydroxide (NaOH). Sodium hydroxide (NaOH): This is a white, solid that is highly soluble in water. It is also known as lye and is used in soapmaking, papermaking, and many other industries.
You could also say Hi and help her and her hydrogen friend find a hydronium pair, that will make her wet (H2O).
On the other hand, OH- represents a hydroxide ion, which is a negatively charged species composed of an oxygen atom bonded to a hydrogen atom with a net charge of -1. Hydroxide ions are formed when a compound or molecule donates or loses a proton (H+) from its hydroxyl group. Hydroxide ions are commonly found in basic or alkaline solutions.
Remember, -HO refers to a hydroxyl group attached to an organic compound, while OH- represents a hydroxide ion with a negative charge.
Why is a hydroxide ion not a double bonded OH? A hydroxide ion is not a double bonded HO because hydrogen atoms cannot form double bonds. Hydrogen atoms only have one electron in their valence shell, and they need two electrons to form a stable bond. A double bond would require hydrogen to share two of its electrons with another atom, but it only has one electron to share.In addition, the electronegativity difference between oxygen and hydrogen is not large enough to support a double bond. Electronegativity is a measure of how strongly an atom attracts electrons. Oxygen is more electronegative than hydrogen, so it pulls the shared electrons in the OH bond closer to itself. This means that the electrons are not shared equally between the two atoms, which is a requirement for a double bond. Therefore, the OH bond in a hydroxide ion is a single bond, not a double bond.
Hydroxide Ion detail
8 Electrons, Polar Molecule, asymmetrical charge
The term "hydroxide" refers to the hydroxide ion. The hydroxide ion is represented as OH- because oxygen has 6 valence electrons and requires 2 additional electrons to complete its octet (8 valence electrons) and achieve a stable electron configuration. Hydrogen, with its single valence electron, can donate that electron to oxygen to form a covalent bond. The resulting molecule is OH, where oxygen has a formal charge of -1 and hydrogen has a formal charge of +1.
When OH gains an additional electron, it becomes the hydroxide ion (OH-), with a net charge of -1. The negative charge is primarily located on the oxygen atom, as it now possesses 7 valence electrons and a stable electron configuration.
In a chemical formula, the minus (-) sign is used to indicate the overall charge of the ion or molecule. In the case of the hydroxide ion, it signifies that the ion carries a single negative charge.
Let's examine the word hydroxide, hydr (hydrogen) and ox - ide, oxygen with suffix "ide"
Hydroxyl Group detail
7 Electrons, Polar Molecule has overall neutral charge
It's important to note that the hydroxyl group in organic compounds is typically neutral and not an ion. The hydroxyl group becomes an ion, known as the hydroxide ion (OH-), when it gains an extra electron to achieve a full negative charge.
The difference in electronegativity between oxygen and hydrogen is not very large, so the OH- group is a weakly polar covalent bond. This means that the electrons are not shared very unequally between the two atoms.
The hydroxyl group, denoted as -OH, is a functional group commonly found in organic compounds. It consists of an oxygen atom bonded to a hydrogen atom. The presence of the hydroxyl group imparts certain chemical properties to the molecule.
The oxygen atom in the hydroxyl group has 6 valence electrons and requires 2 additional electrons to complete its octet (8 valence electrons) for stability. Hydrogen, with its single valence electron, can form a covalent bond with the oxygen atom, resulting in the formation of the hydroxyl group (-OH).
In this molecular arrangement, the oxygen atom in the hydroxyl group retains its high electronegativity, attracting the shared electron pair more strongly than hydrogen. As a result, the oxygen atom carries a partial negative charge (δ-) while the hydrogen atom bears a partial positive charge (δ+). This polarity within the hydroxyl group makes it capable of participating in various chemical reactions, such as hydrogen bonding and nucleophilic substitution.
In summary, the hydroxyl group (-OH) is a functional group in organic chemistry that consists of an oxygen atom bonded to a hydrogen atom. It contributes to the chemical reactivity of molecules and forms the basis of the hydroxide ion (OH-) when it gains an electron to acquire a negative charge.
Acid
Essentially extra protons. Substance that donates H+ ions in water, lowers pH, sour taste, can react with bases to form salts. It has more protons than electrons.
An acid can be defined as a substance that dissociates into one or more hydrogen ions (H+) and one or more negative ions (anions). Thus, an acid can also be defined as a proton (H+) donor.
Cucumbers and vinegar. A women can be called practical and efficient if she use a cucumber like she would use a line – to represent a bond. She saves money, the shape changes, and the change in pH of her "purse" will pickle the cucumber. Some recipes require sweetness to be added, which will occur after she’s finished. Heat helps the cucumber absorb its new slightly acidic environment. Let’s examine this pickle's surface. We have a membrane with channel proteins that will expand and denature based on temperature allowing acid to diffuse across a cucumber's membrane, similar to why your bond – still has a smell after you take a shower. “Let me smell your bond –” lyrics has inherent knowledge most would not anticipate. If your one of those males that look at a cucumber as if it were Barack Obama starring back at you and feel discouraged well there’s a reason she bought more than one cucumber 😉.
An acid is a chemical substance that, when dissolved in water, donates protons (H+) or hydronium ions (H3O+). Acids are characterized by their ability to increase the concentration of hydrogen ions in a solution, resulting in a decrease in pH. They are sour-tasting and can cause a sensation of burning or irritation.
Acids can be categorized into two types:
- Inorganic Acids: acids that do not contain carbon. Examples include hydrochloric acid (HCl), sulfuric acid (H2SO4), and nitric acid (HON3).
- Organic Acids: acids that contain carbon atoms. Examples include acetic acid (CH3COOH), citric acid (C6H8O7), and ascorbic acid (vitamin C).
Acids can react with bases to form salts and water, a process known as neutralization. They also have corrosive properties and can react with certain metals to release hydrogen gas.
Acids have numerous applications in various fields, such as chemical synthesis, food preservation, industrial processes, and scientific research. Additionally, they play essential roles in biological systems and physiological processes, including digestion and cellular metabolism.
Acid washed jeans ... ? Similar to what acid does to your insides it did to the surface of your denim.
She look uncoordinated in those jeans (doing a lot of knee bends and jumps without the hips)? She look like she need electrons? She look like she need something inside? Give her a foundation to build on, be her base. She still walking around like her jeans got a wire frame lining the insides? Electrolysis gets rid of metal, apply electrons (-) and it strips the metal down. Gotta liquidate the insides to speed up the process. Copper sulfate CuSO4 provides electrons and makes elaborate structures just takes time to build something worth looking at, like weeks, unless you do something fancy.
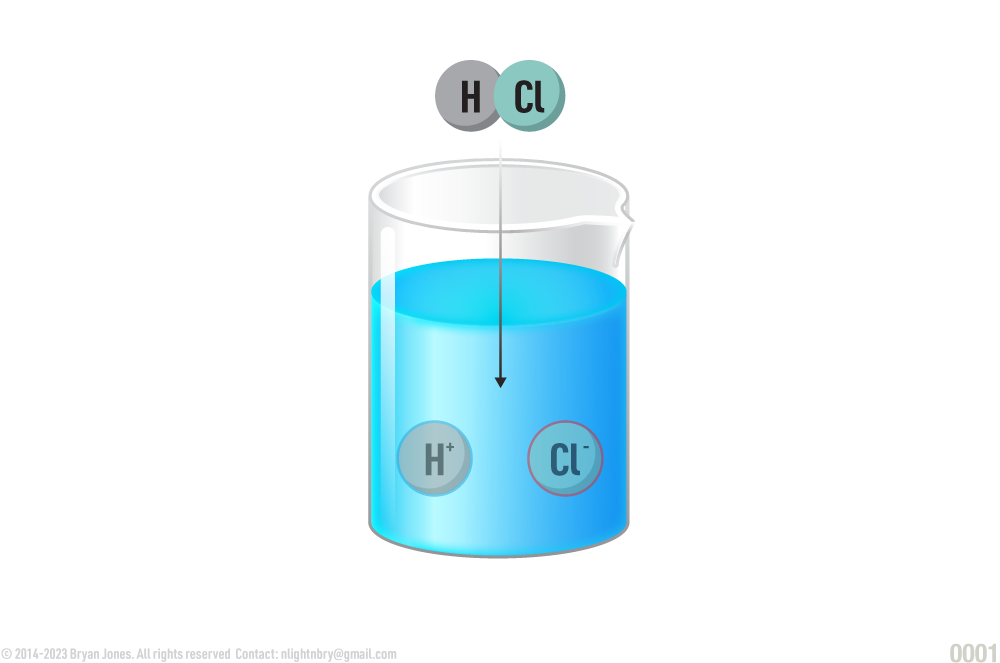
The acid HCl disassociates into H+ and Cl-
Examples of acids
Take your average beer which is ~90% water. In just 10% you feel the entire surface of your mouth respond. Your mouth almost stings, your brain just told you that this substance is acidic. Beer contains a variety of organic acids, including lactic acid, acetic acid, and malic acid. These acids contribute to the tartness of the beer.
The sensations on the sides of your tongue detect sweetness those are the carbohydrates. The full bodied flavor has much to offer, you just have to dunk your tongue in.
There are substances such as alcohol, CO2, Sugars, Flavonoids in this solution that are advantages and your body is letting you know. In just 10% your body detects the smallest sensations. Senses are a complex yet simple mechanisms our bodies have in allowing us to perceive reality. Senses are a result of electric static reactions, depolarization, signal transduction and cascades of neurons that communicate the sum of what you’ve encountered. Channel proteins cause shifts in concentration gradients between one space and another resulting in a cascade, and since acids have extra protons to give, they activate channel proteins scattered across the entire surface of your mouth such as ASICs (acid-sensing ion channels) and others.
Gorgeous woman posing in front of the cereal aisle may make you think of fermentation cycle. Fermentation is the conversation of glucose into pyruvic acid which converts to acetaldehyde then converted further into alcohol this is why when you consume alcohol, your body breaks in down into acetylaldehyde then into pyruvic acid. See Metabolism
Did she bring you a bowl of cereal and a glass of water? Never mix the holes, pick one and stay with it just like one should stay with the women who just brought you breakfast.
Pelvic Pie, Pyruvic acid both give you energy, one of which is more reliable. Pyruvic acid is used as a precursor for Krebs cycle which will become important once your finish eating your cereal.
Give that body a smell, now that the head has dissipated, your left with an aroma, the receptors in your nose activate causing a cascade of electrons, your brain just told me to eat this cereal. If they say Guten Morgen, know they're not inquiring about your relation with Gluten.
Respond by moving head up-down will suffice.
Strong Acid
A strong acid completely breaks apart in water, releasing a high concentration of hydrogen ions (H+). Examples include hydrochloric acid (HCl) and sulfuric acid (H2SO4). They are highly reactive and have a low pH value.
A strong acid is a chemical compound that completely dissociates into its ions when it is dissolved in water. In other words, it ionizes almost completely, releasing a high concentration of hydrogen ions (H+) into the solution. This dissociation process is often represented by a reversible reaction, with the arrow pointing predominantly towards the product side, indicating the completion of the reaction.
The strong acid's ability to dissociate almost entirely is due to its high acidity and the stability of the resulting ions in water. Common examples of strong acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), nitric acid (HNO3), and hydrobromic acid (HBr).
Because of their complete dissociation, strong acids are highly reactive and can readily donate protons (H+) to other substances, making them effective for various chemical reactions, such as neutralization reactions and pH adjustments. Strong acids typically have a pH value less than 2 when dissolved in water, indicating their high acidity.
Weak Acid
A weak acid is a type of acid that only partially dissociates or ionizes in water, resulting in a relatively low concentration of hydrogen ions (H+) in the solution. In other words, when a weak acid is dissolved in water, it does not completely break apart into ions. Instead, it exists in equilibrium with its dissociated and undissociated forms.
The dissociation of a weak acid can be represented by the following general equation:
HA (aq) ⇌ H+ (aq) + A- (aq)
In this equation, HA represents the undissociated form of the weak acid, while H- represents the hydrogen ions and A- represents the corresponding conjugate base that are formed when the weak acid partially dissociates.
The extent to which a weak acid dissociates in water is quantified by its acid dissociation constant (Ka). A smaller value of Ka indicates a weaker acid. Weak acids have Ka values less than 1, whereas strong acids have Ka values much greater than 1.
Examples of weak acids include acetic acid (CH3COOH), formic acid (HCOOH), and carbonic acid (H2CO3). These acids release relatively few hydrogen ions into solution compared to strong acids like hydrochloric acid (HCl) or sulfuric acid (H2SO4).
Acid equilibrium
Acid equilibrium is the balanced state between an acid and its conjugate base, where both the dissociation and reformation of the acid occur simultaneously at a constant rate. It is characterized by the acid dissociation constant (Ka) and involves reversible reactions.
Acid equilibrium refers to the state of balance or equilibrium that exists between an acid and its conjugate base in a chemical system. It involves the reversible reaction between the dissociated (ionized) form of an acid and its conjugate base, as well as the backward reaction where the acid is re-formed from the ions.
In an acid equilibrium, the forward and backward reactions occur simultaneously, but at a constant rate. This results in a stable concentration of both the acid and its conjugate base in the solution. The equilibrium is governed by the principles of Le Chatelier's principle and the acid dissociation constant (Ka).
The acid equilibrium can be represented by the following generic equation:
HA (acid) ⇌ H+ (hydrogen ion) + A- (conjugate base)
In this equation, HA represents the acid in its undissociated form, while H+ represents the hydrogen ions and A- represents the conjugate base formed when the acid dissociates.
The acid equilibrium is characterized by the equilibrium constant, known as the acid dissociation constant (Ka), which represents the ratio of the concentration of the products (H+ and A-) to the concentration of the reactant (HA). The Ka value provides information about the strength of the acid. A larger Ka value indicates a stronger acid with a greater tendency to dissociate.
Understanding acid equilibrium is crucial in various chemical processes, such as acid-base reactions, buffer systems, and pH regulation in biological systems.
Lewis Acid
Lewis acid: Electron pair acceptor.
A Lewis acid is a chemical species that can accept a pair of electrons to form a covalent bond. It is defined within the Lewis acid-base theory, proposed by Gilbert N. Lewis. In this theory, an acid is any chemical species that can receive or accept an electron pair, while a base is a species that can donate or provide an electron pair.
Unlike traditional acid-base theories that focus on proton transfer, the Lewis acid-base theory is more general and encompasses a broader range of reactions. According to this theory, a Lewis acid can be a molecule, ion, or atom that has an electron-deficient center capable of accepting an electron pair.
Some examples of Lewis acids include metal ions (such as Al3+, Fe3+), electron-deficient molecules (such as boron trifluoride, BF3), and transition metal complexes.
Lewis acids play a fundamental role in various chemical reactions, such as coordination chemistry, catalysis, and organic synthesis. They can react with Lewis bases to form coordinate covalent bonds, where the Lewis acid accepts a pair of electrons from the Lewis base.
Polyprotic acid
Polyprotic acid refers to an acid that has more than one ionizable hydrogen atom or proton, which can be successively released in separate stages or steps when dissolved in water. Each step corresponds to a different equilibrium reaction with its own equilibrium constant and represents the successive removal of a proton from the acid molecule. Examples of polyprotic acids include sulfuric acid (H2SO4) and phosphoric acid (H3PO4).
Base
Essentially extra electrons. A base is a molecule which dissociates into negative ion(s). It has more electrons than protons.
A base dissociates into one or more positive ions (cations) plus one or more negatively charged hydroxide ions (OH-) that can accept or combine with protons. Sodium hydroxide (NaOH) is a base because it dissociates to release OH-, which has a strong attraction for protons and is among the most important proton acceptors.
The base NaOH disassociates into Na+ and OH--

A base, also known as an alkali, is a chemical substance that, when dissolved in water, accepts protons (H+) or donates hydroxide ions (OH-). Bases are characterized by their ability to increase the concentration of hydroxide ions in a solution, resulting in an increase in pH.
Bases can be classified into two types:
- Inorganic Bases: bases that do not contain carbon. Examples include hydroxides of alkali metals (such as sodium hydroxide, NaOH) and alkaline earth metals (such as calcium hydroxide, Ca(OH)2).
- Organic Bases: bases that contain carbon atoms. Examples include amines (such as ammonia, NH3) and organic compounds with amino groups (such as methylamine, CH3NH2).
Bases have certain characteristic properties, such as a bitter taste, a slippery or soapy feel, and the ability to neutralize acids. When a base reacts with an acid, a chemical reaction called neutralization occurs, resulting in the formation of a salt and water.
Bases find applications in various fields. They are commonly used in cleaning products, such as soaps and detergents, due to their ability to emulsify fats and oils. Bases are also utilized in chemical reactions, laboratory procedures, and as ingredients in medications and personal care products.
In biological systems, bases play important roles. For example, in the human body, bases like bicarbonate ions (HCO3)- help regulate pH and maintain the acid-base balance in blood and other bodily fluids.
Examples of bases
Strong Base
A strong base is a substance that completely dissociates in water to release hydroxide ions (OH-) and has a high ability to increase the pH of a solution.
strong base is a substance that completely dissociates or ionizes in water to release hydroxide ions (OH-) as the sole reactive species. It is a compound that has a high affinity for protons (H+) and readily accepts them to form water molecules. In other words, strong bases are highly effective at removing hydrogen ions from a solution.
Strong bases have a high concentration of hydroxide ions and are capable of rapidly increasing the pH of a solution. They are typically composed of metal cations combined with hydroxide anions, such as sodium hydroxide (NaOH) and potassium hydroxide (KOH-).
When a strong base is dissolved in water, it dissociates completely, producing hydroxide ions. For example:
NaOH- (s) → Na+ (aq) + OH- (aq)
The hydroxide ions then interact with water molecules to increase the concentration of hydroxide ions and decrease the concentration of hydrogen ions, resulting in a basic (alkaline) solution.
Strong bases are commonly used in various applications, including in chemical synthesis, manufacturing processes, and as alkaline reagents in laboratories. They are known for their ability to neutralize acids and participate in acid-base reactions.
Base equilibrium
Base equilibrium is a balanced state between a base and its conjugate acid, involving reversible proton transfer reactions.
Base equilibrium refers to the state of balance or equilibrium that exists between a base and its conjugate acid in a chemical system. It involves the reversible reaction between the base accepting a proton (H+) and forming its conjugate acid, as well as the backward reaction where the conjugate acid can release a proton to regenerate the base.
In a base equilibrium, the forward and backward reactions occur simultaneously but at a constant rate, resulting in a stable concentration of both the base and its conjugate acid in the solution. The equilibrium is governed by the principles of Le Chatelier's principle and the base dissociation constant (Kb).
The base equilibrium can be represented by the following generic equation:
B (base) + H2O ⇌ BH+ (conjugate acid) + OH- (hydroxide ion)
In this equation, B represents the base, H2O represents water, BH+ represents the conjugate acid formed when the base accepts a proton, and OH- represents the hydroxide ion that can be released by the conjugate acid.
The base equilibrium is characterized by the equilibrium constant, known as the base dissociation constant (Kb), which represents the ratio of the concentration of the products (BH+ and OH-) to the concentration of the reactant (B). The Kb value provides information about the strength of the base. A larger Kb value indicates a stronger base with a greater tendency to accept protons.
Understanding base equilibrium is important in various chemical processes, including acid-base reactions, buffer systems, and pH regulation in biological systems.
Weak Base
Weak bases are substances that partially ionize or react with water to release a relatively low concentration of hydroxide ions (OH-) in a solution. They do not completely dissociate and exist in equilibrium with their undissociated and dissociated forms. Examples include ammonia (NH3) and organic amines.
Weak bases are substances that exhibit limited ionization or reaction with water, resulting in a relatively small concentration of hydroxide ions (OH-) in a solution. Unlike strong bases, weak bases do not fully dissociate and exist in equilibrium between their undissociated form and the ions formed upon partial dissociation.
When a weak base is dissolved in water, only a fraction of the molecules react with water to generate hydroxide ions. This partial ionization is represented by the following general equation:
B (base) + H2O ⇌ BH+ (conjugate acid) + OH- (hydroxide ion)
In this equation, B represents the weak base, H2O represents water, BH+ represents the conjugate acid formed when the weak base accepts a proton, and OH- represents the hydroxide ion.
The extent to which a weak base ionizes in water is quantified by its base dissociation constant (Kb). A smaller Kb value indicates a weaker base. Weak bases have Kb values less than 1, whereas strong bases have Kb values much greater than 1.
Examples of weak bases include ammonia (NH3), organic amines (such as methylamine, CH3NH2), and carbonate ions (CO32-). These substances have a limited ability to accept protons and generate hydroxide ions compared to strong bases like sodium hydroxide (NaOH) or potassium hydroxide (KOH).
In summary, weak bases are substances that only partially ionize in water, resulting in a lower concentration of hydroxide ions. They exist in equilibrium between their undissociated and dissociated forms and have smaller base dissociation constants compared to strong bases.
Lewis Base
Lewis base: Electron pair donor.
A Lewis base is a chemical species that can donate a pair of electrons to form a covalent bond. It is defined within the Lewis acid-base theory proposed by Gilbert N. Lewis. According to this theory, a Lewis base is any species that can provide or donate an electron pair.
Unlike traditional acid-base theories that focus on proton transfer, the Lewis acid-base theory is more general and encompasses a wider range of reactions. In this theory, a Lewis base can be a molecule, ion, or atom that has an electron-rich center capable of donating an electron pair.
Some examples of Lewis bases include lone pair donors such as ammonia (NH3), water (H2O), and hydroxide ions (OH-). These species can interact with Lewis acids, which are electron pair acceptors, to form coordinate covalent bonds. In the process, the Lewis base donates its electron pair to the Lewis acid.
Lewis bases play a fundamental role in various chemical reactions, including coordination chemistry, catalysis, and organic synthesis. They are involved in forming complex ions, participating in acid-base reactions, and acting as nucleophiles in substitution or addition reactions.
Salt
A salt dissociates into positive ions and negative ions
All salts are made of ionic bonds. Ionic bonds are formed between positively charged ions (cations) and negatively charged ions (anions). In salts, the cations and anions are held together by these strong electrostatic forces of attraction, creating a stable crystal lattice structure. This ionic bonding is what gives salts their characteristic properties, such as high melting point and solubility in water.
There are several varieties of salts. Salts that hydrolyze to produce hydroxide ions when dissolved in water are basic salts, while those that hydrolyze to produce hydronium ions in water are acidic salts. Neutral salts are those that are neither acid nor basic salts.
(x)Is she salty, or being salty?
(x)How did she get so salty?
(–)These are all good questions.
(x)She's out running and she comes back huffing and puffing..What do you do?
(–)Put her out under the sun. After she dries out, it's time to get your electrolytes.
(–)Give her a smell, doesn't she smell fresh? All that salty sweat changed the composition of her skin (epidermis). It's one reason fish are packaged with salt, another is that salt can destroy bacteria, pulls those tampon looking bacteria apart. Those single-celled flagella twirling bacteria and their antigens are a reason she went for that run in the first place?
Salt changes the pH of her skin, creates an environment unfavorable for bacteria and fungal growth. She needs to dry, give her a spin, make sure she's golden brown, other wise she'll want an epson salt bath. A moist environment can promote growth of certain microorganisms. Excessive sweating can contribute to the proliferation of these organisms.
NaCl, a salt disassociates into Na+ and Cl-
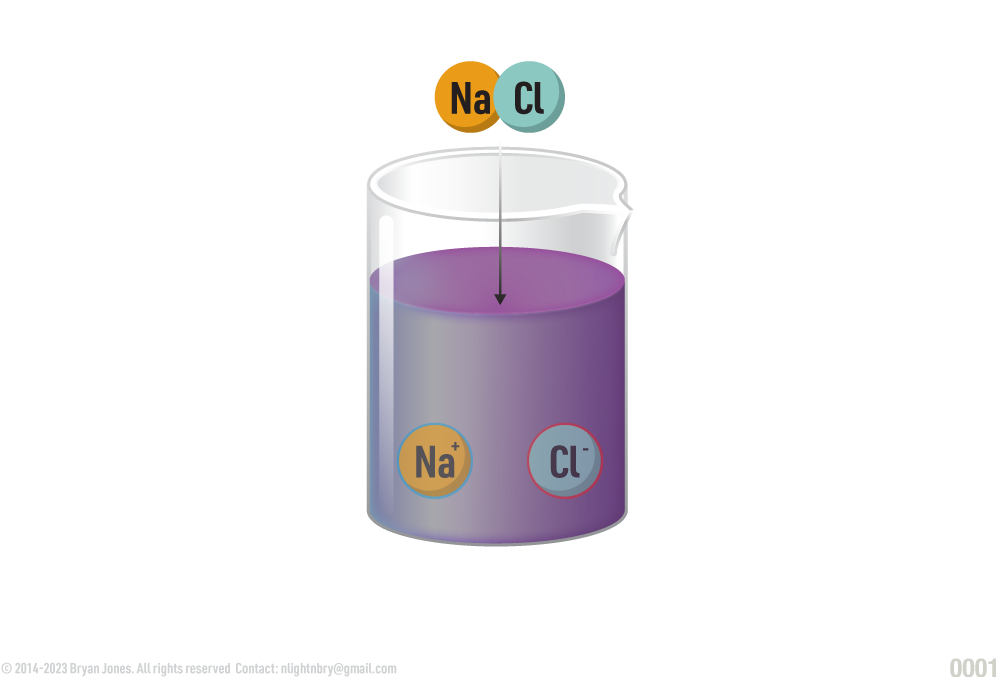
In chemistry, the term "salt" refers to a compound composed of positive and negative ions that are held together by electrostatic forces. Salts are formed through a chemical reaction known as neutralization, which occurs when an acid reacts with a base.
The basic structure of a salt consists of a positively charged ion, known as a cation, and a negatively charged ion, known as an anion. The cation is typically a metal or a positively charged polyatomic ion, while the anion is usually a nonmetal or a negatively charged polyatomic ion.
Salts are generally solid crystalline compounds, but some can also be found in liquid or gaseous states. They exhibit various physical properties such as high melting points, solubility in water, and electrical conductivity when dissolved in water or melted.
Salts have a wide range of applications in various industries and everyday life. They are commonly used as seasoning agents in food, as preservatives, in chemical synthesis, in pharmaceuticals, and as electrolytes in batteries and other electrochemical devices.
Examples of salts include sodium chloride (table salt, NaCl), calcium carbonate (chalk, CaCO3), potassium nitrate (saltpetre, KNO3), and ammonium sulfate ((NH4)2SO4).
Acid-Base Balance and the Concept of pH
The pH of a solution is a measure of its strength along a continuum from acid to base. 0 is a near-maximum for acids, 7 is considered neutral pH and 14 is a near maximum for bases.
The pH of a solution is a logarithmic scale used to quantify the acidity or basicity of a substance. It represents the concentration of hydrogen ions (H+) present in the solution. The scale ranges from 0 to 14, with 7 being neutral. Values below 7 indicate increasing acidity, with 0 representing a near-maximum for acids, while values above 7 indicate increasing alkalinity or basicity, with 14 representing a near-maximum for bases.
You got your Basic betches, and Acids. Acid can be fun, don't do too much though, if you're fully hydrated then you're already slightly acidic, it's probably why you have the giggles.
Being basic isn't bad, it's a stable choice, you can build a life on it. It's a healthy choice, less chance of disease. Less proliferation of broken cells causing havoc in your body. Nothing wrong with basic it's why Nucleic acids and Amino acids are able to function. We all know Acids are more fun. For example Alcohol, it even has HO in it. Acidic, that's like dic-I-ace in some minds.
Too much alcohol causes a build-up of acetaldehyde which comes from the break down of alcohol and leads to a sick, awful feeling when it builds up in the body known as a hang-over. A solution to aid in the breakdown of acetaldehyde is to consume L-cysteine, an Amino acid which helps in the production of another compound that breaks down acetaldehyde. Look on the back of your protein mix and see if it's there, do not make yourself a large shake. Consume like 1/4 of what you normally would, your kidneys are already working overtime to breakdown the alcohol. This is when planning ahead and drinking responsibly should be exercised. While you still can!
pH scale:
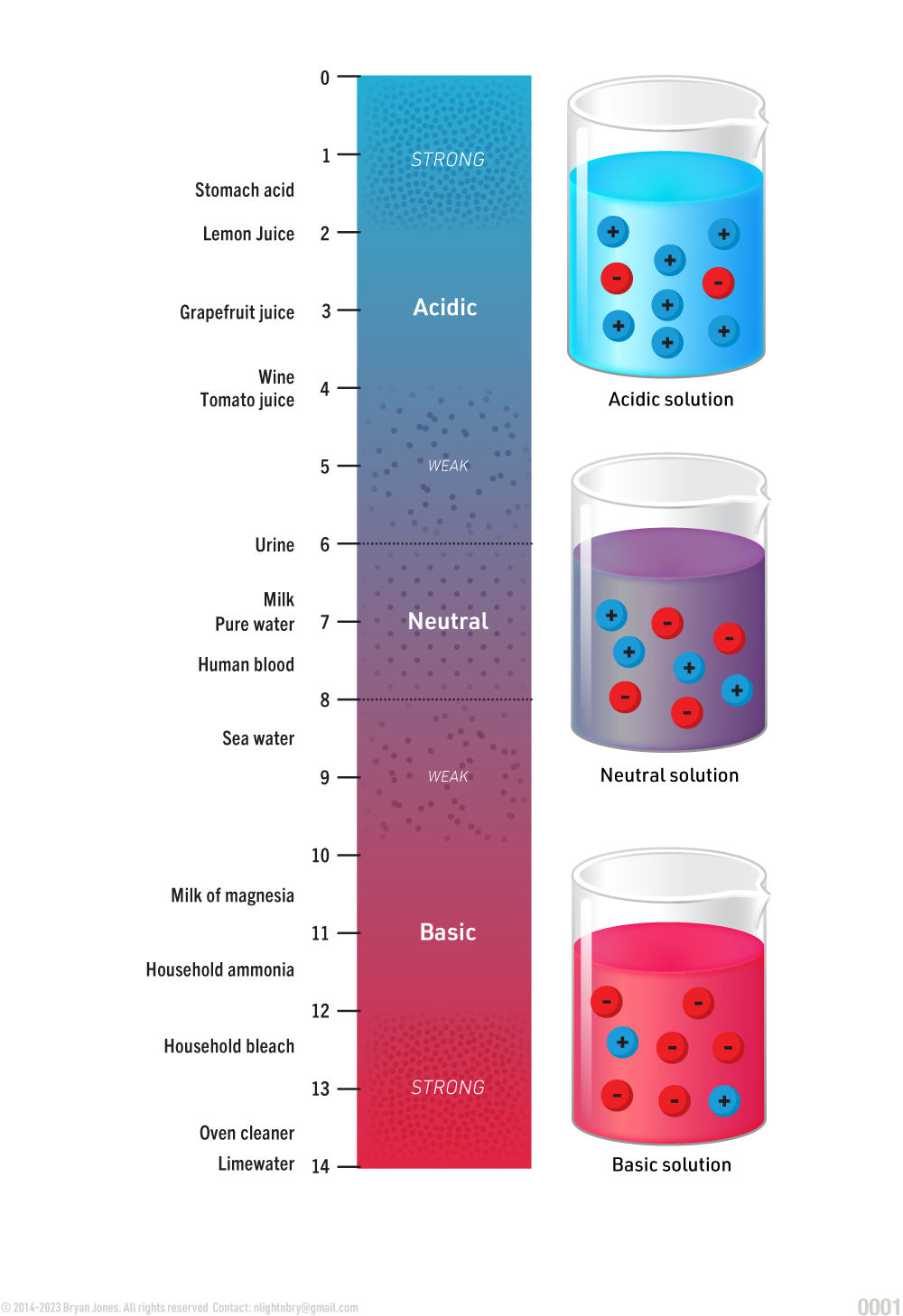
The pH scale provides a quantitative measure that allows us to compare the relative strengths of acidic and basic solutions. Each unit on the pH scale represents a tenfold difference in the concentration of hydrogen ions. Therefore, a solution with a pH of 4 is ten times more acidic than a solution with a pH of 5 and a hundred times more acidic than a solution with a pH of 6.
It's important to note that the pH scale is logarithmic, meaning that each whole number change on the scale represents a tenfold difference in acidity or basicity. For example, a solution with a pH of 3 is ten times more acidic than a solution with a pH of 4, and a hundred times more acidic than a solution with a pH of 5.
The pH scale is a logarithmic measurement used to describe the acidity or basicity of a solution. It ranges from 0 to 14, with 7 representing neutrality, values below 7 indicating acidity (with 0 being a near-maximum for acids), and values above 7 indicating alkalinity or basicity (with 14 being a near-maximum for bases). The scale allows for easy comparison of the relative strengths of acidic and basic solutions based on the concentration of hydrogen ions.
Buffers
A Buffer range is like a shooting range, or a group in the way. If you concentrate enough you can overcome them. A buffer is also the rebound boyfriend, or girlfriend.
Buffers such as water provide a protective cushion for organisms. A buffer prevents drastic changes in pH, this enables organisms to gradually change their overall pH and absorb excess heat in which a cell isn't able to immediately compensate for. This is why astrobiologists look for water when considering if a planet could harbor life. A buffer range is the area in which no noticeable changes occur in pH. Oceans on our planet absorb enormous amounts of CO2 and heat because the H2O in these oceans act as a planetary buffer.
Yeast, bakers yeast that is can act as a pseudo buffer against alcohol, but if you're not a trained scientist or expert it can mess you up even further, in fact just glance past that statement and focus on something else, like the graph below, pretty two lines and a gradient, a buffer? is that like when you have a bunch of side chicks? (–) Yes, that's exactly correct. They do buffer sad-feelings you get from just one ... solution, but if they're acidic or basic enough they can overcome all those side choices.
Acid-base Buffer Relation
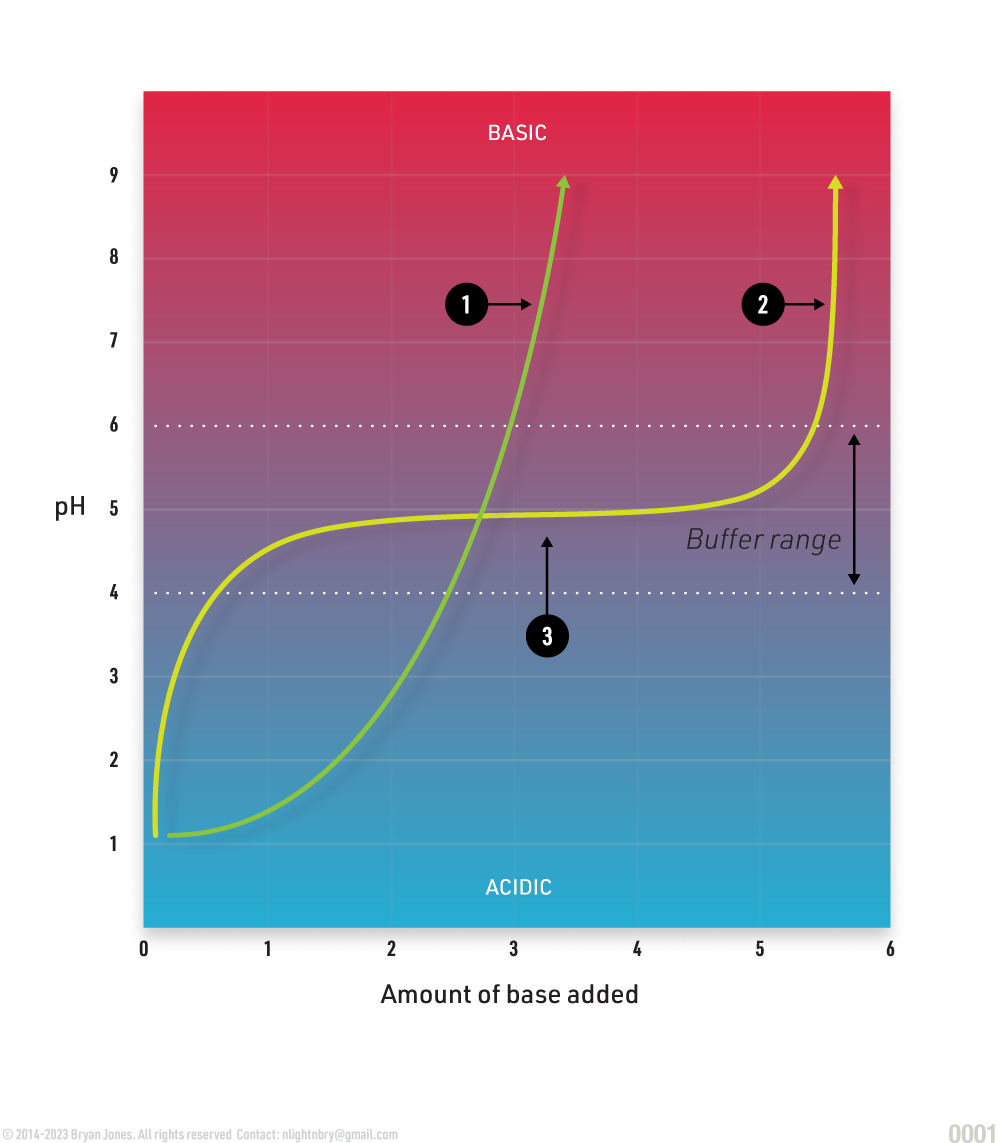
Exorcism? is what it feels like the following day, is when all you want is to get this out of me, coincidently monks hand wrote all those religious documents and were great brewers, all those cups of wine. wafers and wine, snack time.

There is a rapid increase in pH as base is added due to absence of buffer

When buffer capacity is exceeded, adding additional substance greatly increases pH.

Substances in the presence of buffer. When additions of large quantities of base result in relatively small changes in pH.
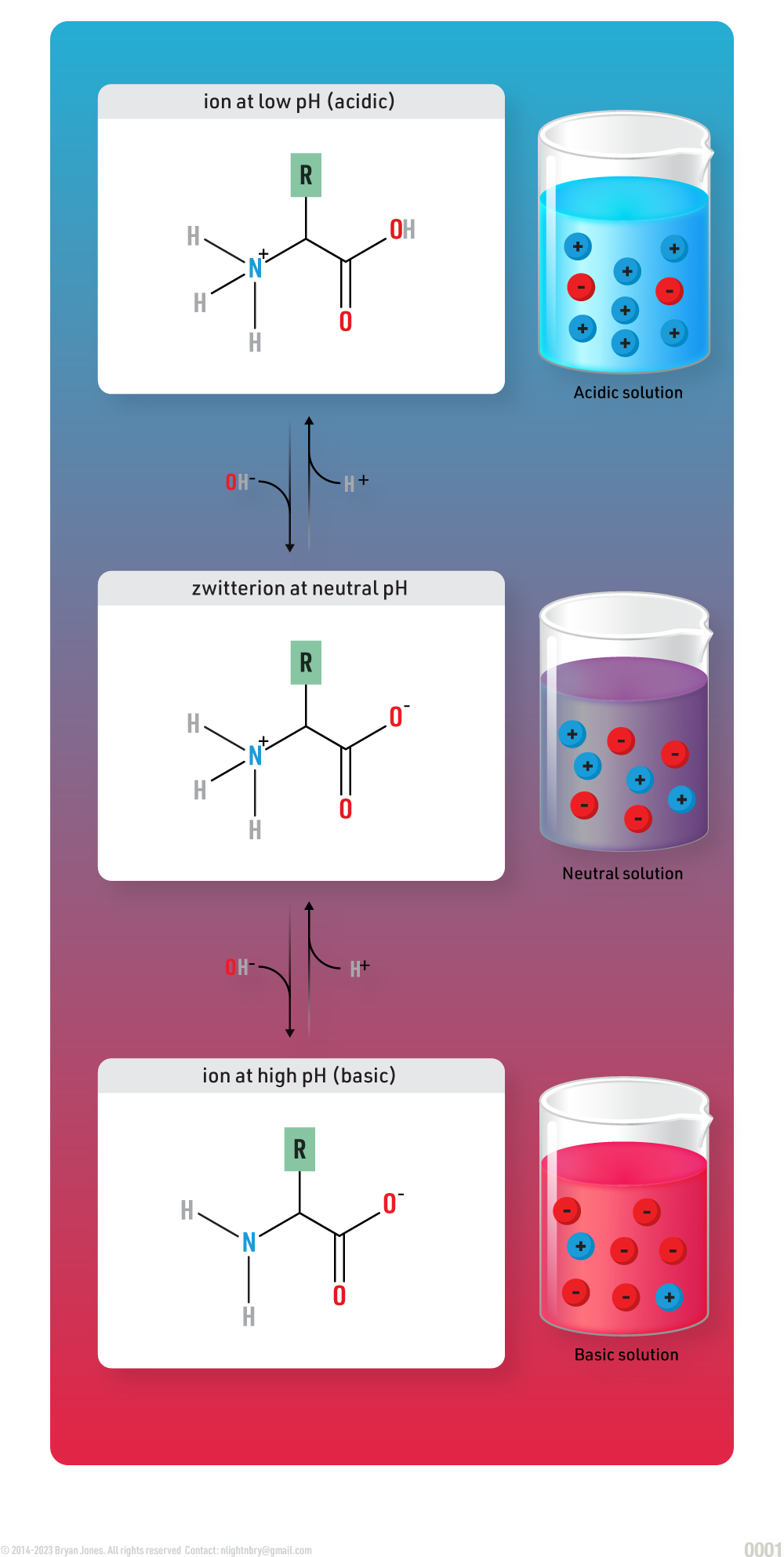
Amphoteric Substance
An amphoteric substance is a chemical compound or species that can exhibit properties of both an acid and a base.
It has the ability to react with both acids and bases, depending on the circumstances. In an acidic environment, an amphoteric substance can behave as a base by accepting protons (H+). Conversely, in a basic environment, it can act as an acid by donating protons. This dual behavior arises from the presence of reactive sites or functional groups in the molecule that can either accept or donate protons. Examples of amphoteric substances include water (H2O) and amino acids.
A zwitterion is an example of an amphoteric substance. A zwitterion is a molecule or ion that contains both positively and negatively charged functional groups, resulting in a neutral overall charge. It possesses an acidic group that can donate a proton (act as an acid) and a basic group that can accept a proton (act as a base). This dual nature allows zwitterions to exhibit amphoteric behavior, reacting as both an acid and a base depending on the pH of the surrounding environment. Amino acids, such as glycine, are prime examples of zwitterions.
Zwitterion Structure of an Amino acid: