Carbohydrates
Carbohydrates are one of the main classes of biological molecules that provide energy for the body's metabolic processes. They are made up of carbon, hydrogen, and oxygen atoms and can be found in many foods such as fruits, vegetables, grains, and dairy products.
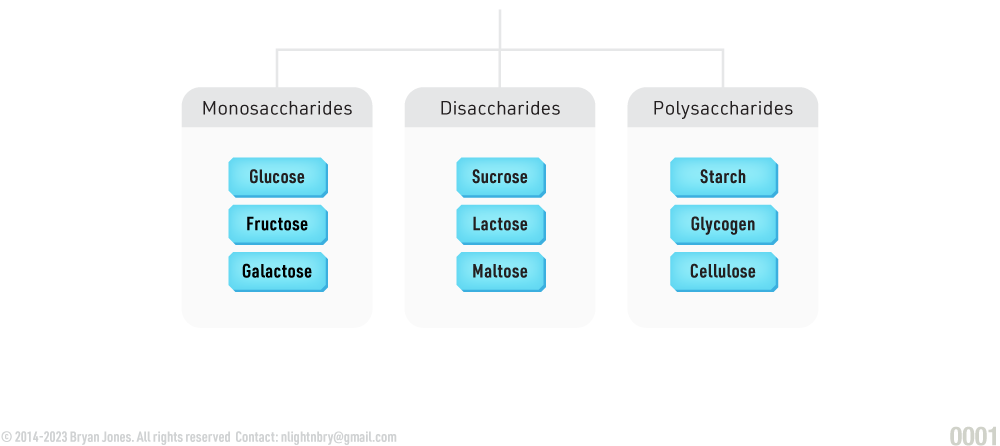
Carbohydrates is a group containing monosaccharides, disaccharides, and polysaccharides. Monosaccharides are the smallest units, disaccharide molecules have two monosaccharide units. Chains with many monosaccharides joined together are called polysaccharides. Polysaccharides can be hydrolyzed back to their constituent monosaccharides.
What is a Carbohydrate?
A carbohydrate is a source of energy.
Carbohydrates can be classified into several different types based on their chemical structure, including monosaccharides, disaccharides, and polysaccharides.
- Monosaccharides: These are the simplest form of carbohydrate, consisting of a single sugar molecule such as glucose, fructose, or galactose.
- Disaccharides: These are made up of two monosaccharides linked together, such as sucrose (table sugar), lactose (milk sugar), and maltose (found in grains).
- Polysaccharides: These are long chains of monosaccharides linked together, such as starch (found in grains and vegetables), glycogen (found in the liver and muscles), and cellulose (found in plant cell walls).
Generalized Symbol

Carbohydrates provide energy to the body by breaking down into glucose, which can be used by cells for fuel. Excess glucose is stored in the liver and muscles as glycogen, which can be converted back into glucose as needed. Carbohydrates also play a role in the structure of cells and tissues, as well as in communication between cells.
It's important to note that not all carbohydrates are created equal, and some sources are healthier than others. Complex carbohydrates, such as whole grains and vegetables, provide more fiber and nutrients than simple carbohydrates, such as refined sugars and processed foods. Eating a balanced diet that includes a variety of carbohydrates can help to maintain overall health and prevent chronic diseases such as diabetes and heart disease.
Carbohydrates are used to build many components of cells such as membranes and cell walls. Carbohydrates are also used in junction with other compounds such as nitrogenous bases to create nucleotides which are the monomers of DNA and RNA. Carbohydrates are utilized in production of amino acids and lipids.
Carbohydrates account for 60-65% or our daily food intake. We also use them for clothing (cotton, linen, rayon), shelter (wood) and extending our ability to remember (paper). Our species and other animals cannot synthesize carbohydrates from carbon dioxide and water and are therefore dependent on the plant kingdom to provide these resources. Carbohydrates are classified in IUPAC terms either a polyhydroxy aldehydes or a polyhydroxy ketone. Our bodies can remove the functional group from carbohydrates and swop it with another function group if needed.
What are Carbohydrates Made of?
Monosaccharides, disaccharides, polysaccharides
Two or more monosaccharides can link together to form chains that contain from two to several hundred or thousand monosaccharide units. Prefixes are used to indicate the number of such units in the chains.
Disaccharide molecules have two monosaccharide units. Polysaccharides consist of chained units with many monosaccharides joined (bonded) together. All disaccharides and polysaccharides can be hydrolyzed back to their constituent monosaccharides.
Carbohydrates
The Structure of a Carbohydrate:
Hydrogen, Carbon, Oxygen
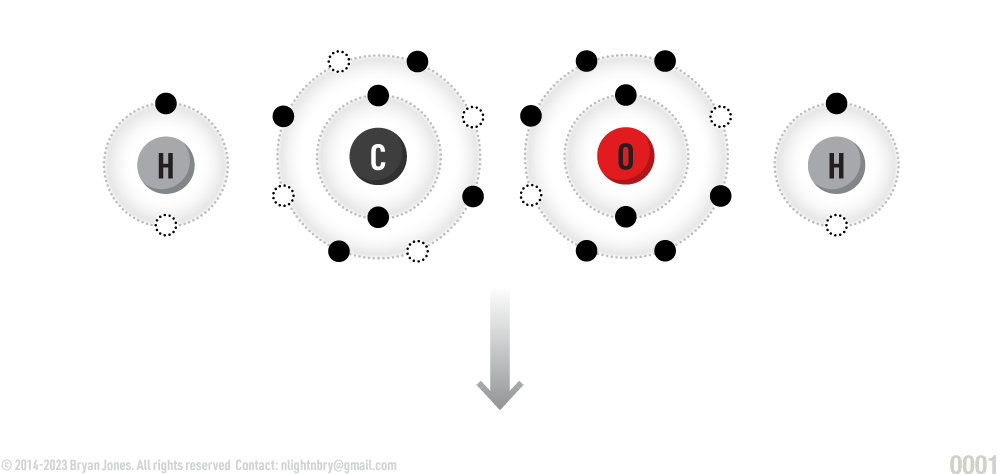
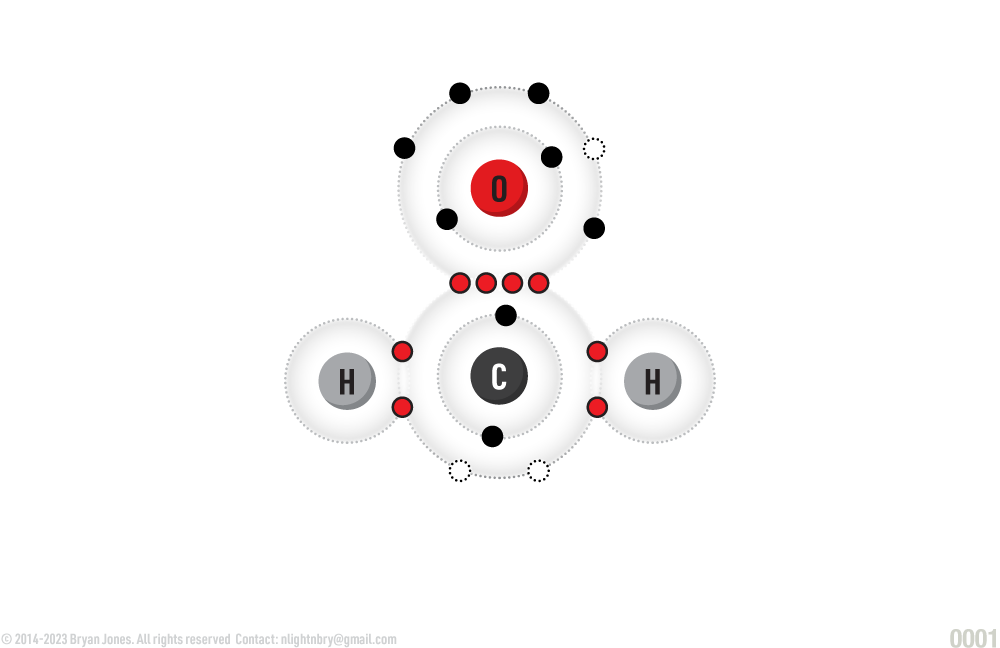
Carbohydrates (also called saccharides) are molecular compounds made from three elements: carbon (C), hydrogen (H) and oxygen (O) in a ratio of 1:2:1 for example (CH2O)n , where n is greater than or equal to 3, 4, 5, 6, or 7.
Atoms combine to form a CH2O molecule (carbohydrate)
Monosaccharides
The smallest carbohydrates
Monosaccharides are carbohydrates, a major source of energy for metabolism, used in biosynthesis where substances are converted to more complex products. Carbohydrates are also used to dismantle complex products when they're not in need. Catabolic and Anabolic reactions will be discussed in Metabolism chapter.
Monosaccharide is the designation for carbohydrates in their simplest form-those that cannot be hydrolyzed to produce even smaller carbohydrates are called monosaccharides, such a fructose and glucose. Two or more monosaccharides can link together to form chains that contain from two to several hundred or thousand monosaccharide units. Prefixes are used to indicate the number of such units in the chains.
Structural Formula: Monosaccharide (Glucose)

Classes of Monosaccharides:
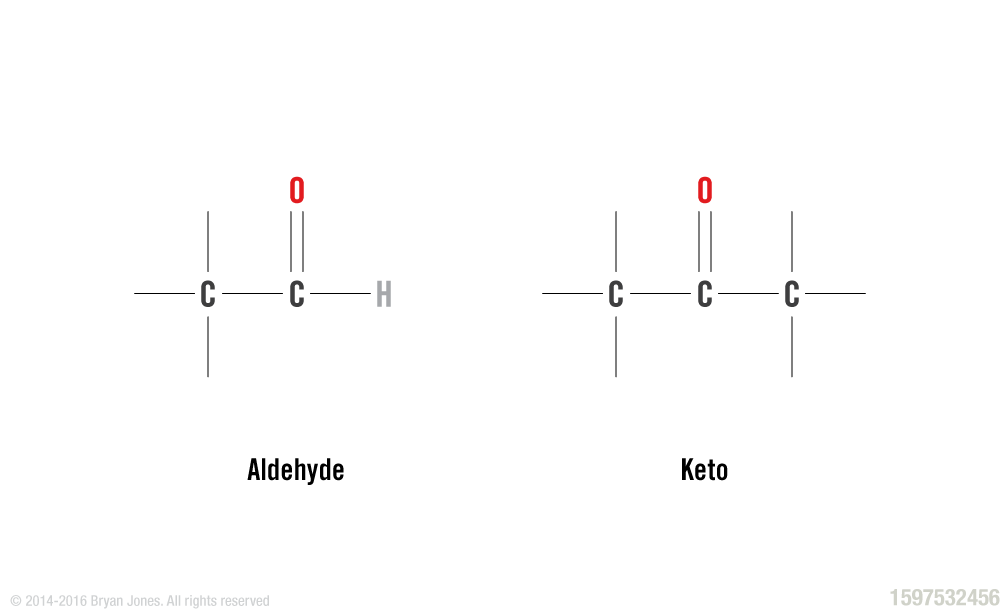
Monosaccharides are covalently bonded combinations of carbon and water in a one-to-one ratio. The most common monosaccharides are pentoses containing 5 carbon atoms and hexoses which contain 6 carbon atoms. All monosaccharides contain hydroxyl (–OH) groups and either an aldehyde or a keto group.
Some compounds are used more than others because they’re easier to use. Their “anatomical structure” that is their chemical structure is arranged in such a way that our metabolic pathways can break those bonds faster and more efficient than others such as high-fructose corn syrup.
Structural Formula: Aldehyde and Keto groups
"Keto" is a term commonly used to refer to the ketogenic diet or ketosis. The ketogenic diet is a low-carbohydrate, high-fat diet that aims to shift the body's metabolism into a state called ketosis. In ketosis, the body primarily relies on fat for fuel instead of carbohydrates. By drastically reducing carbohydrate intake and increasing fat consumption, the body is forced to break down stored fat into molecules called ketones, which can be used as an alternative energy source by the brain and other tissues, which can also help break down High-fructose as your body searches for energy. Your body has various genes that designate where to allocate extra energy and the various micro-macro structures can be unappealing, your best option is to reduce consuming foods that your body accumulates.
proteins and lipids while thinking about bread because carbs are your bodies fastest means of producing energy which is why you crave them. Your body builds all these enzymes to breakdown glucose, but your body has to produce enzymes just to convert fructose into glucose before it can be used as energy which is one reason it accumulates in the body.
D-Glucose is the principal external source of energy for most cells and can exist in three different forms: a linear structure and two different hemiacetal ring structures.
Linear and Hemiacetal ring structures:
Structural Formula: D-Glucofuranose, D-Glucose
Structural Formula: D-Glucopyranose, D-Mannose, D-Galactose
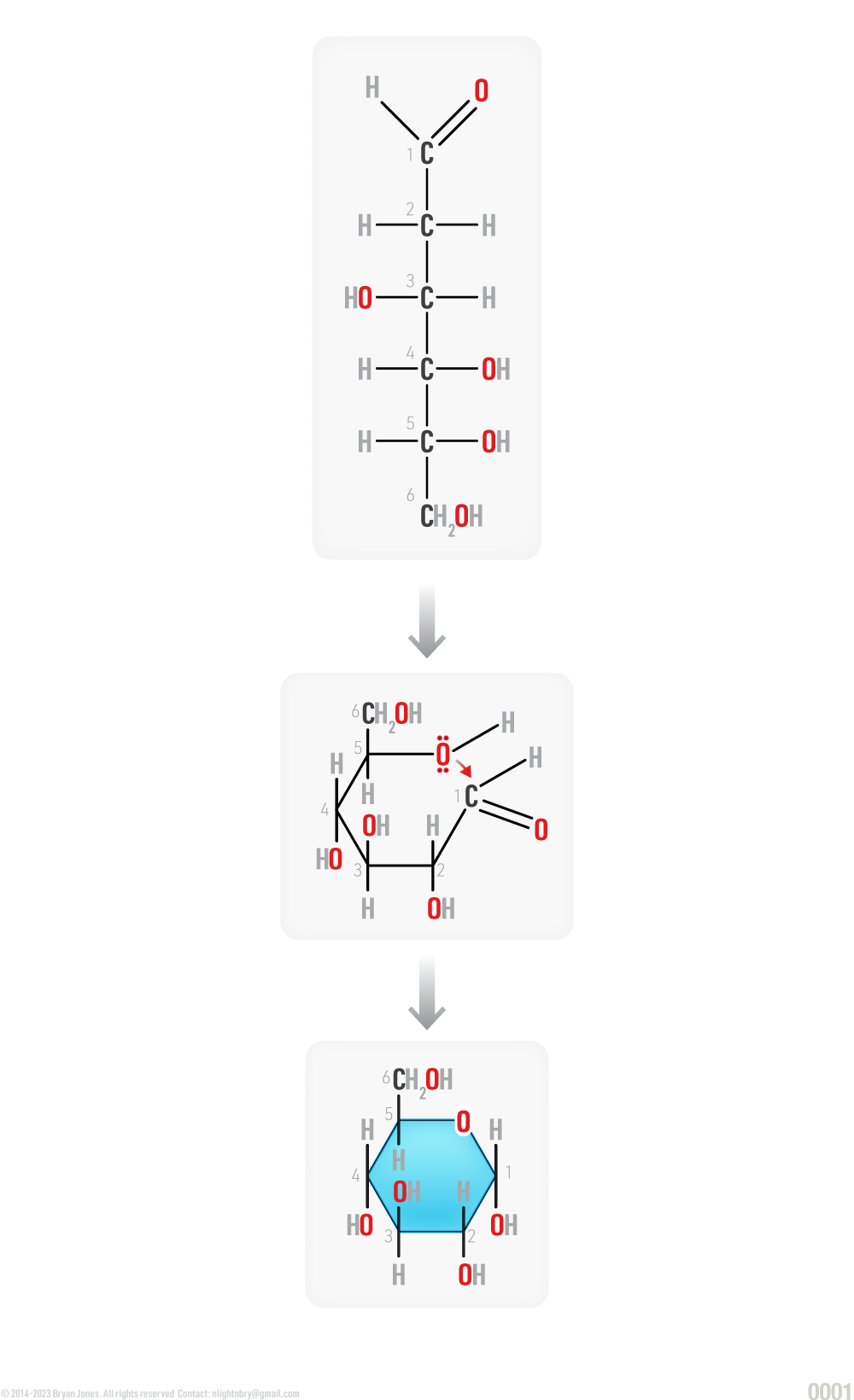
If the aldehyde group on carbon 1' reacts with the hydroxyl group on carbon 5' on the linear structure of D-Glucose, D-glucopyranose (C6H12O6 ), consisting of a six member ring, called a hemiacetal. The Greek word hèmi means half.
Structural Formula: Glucose changes to D-glucopyranose
Cyclic Structures of Monosaccharides:
Cyclization of D-Glucose
Properties of Monosaccharides:
Monosaccharides such as glucose and fructose are crystalline solids at room temperature, soluble in water, and each molecule has several -OH hydroxyl groups that readily engage in hydrogen bonding. The chemical behavior of these monosaccharides is determined by their functional groups. Carbs are pennies, and $100 are polysaccharides
Disaccharides
Disaccharides are formed from two monosaccharides.
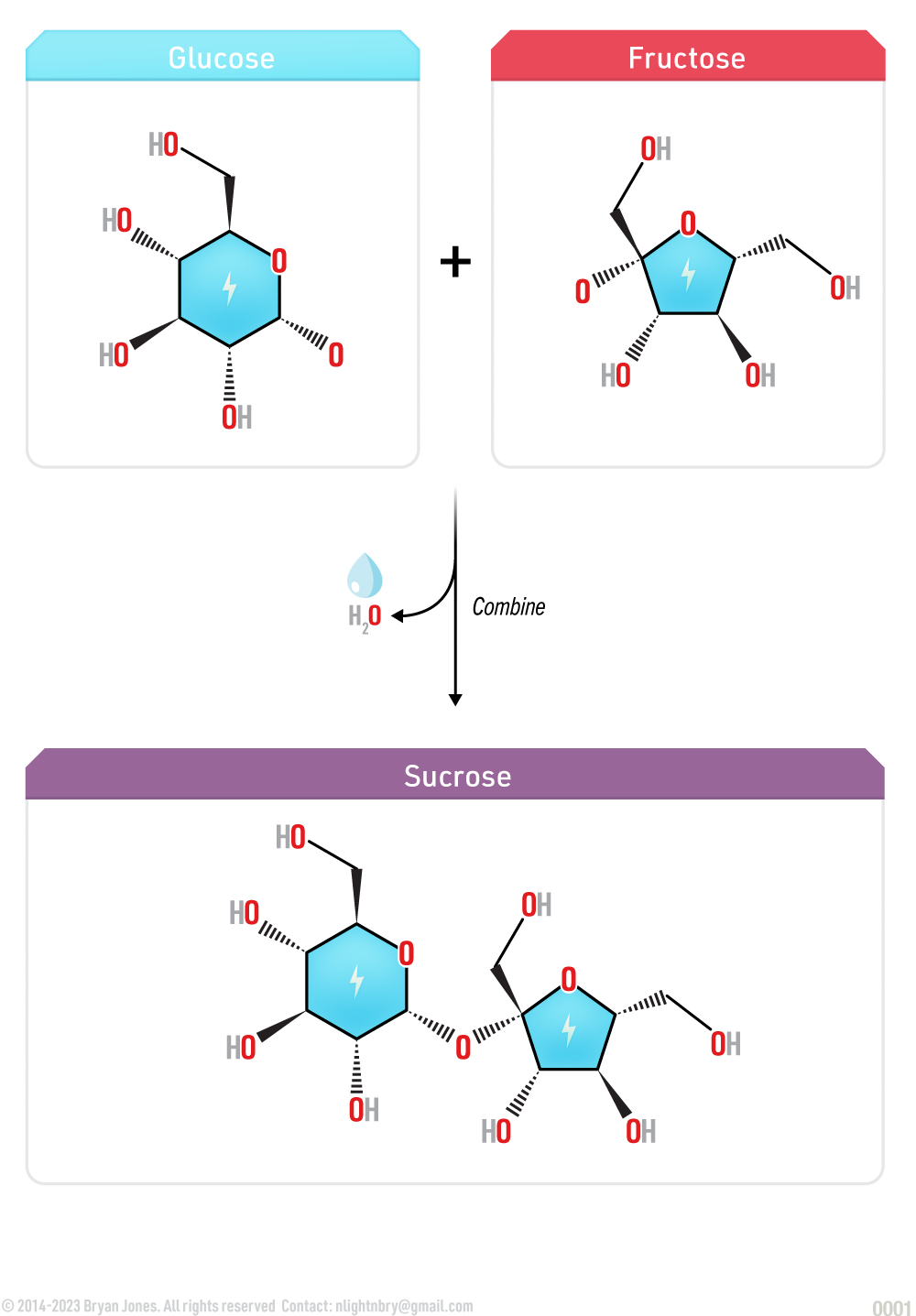
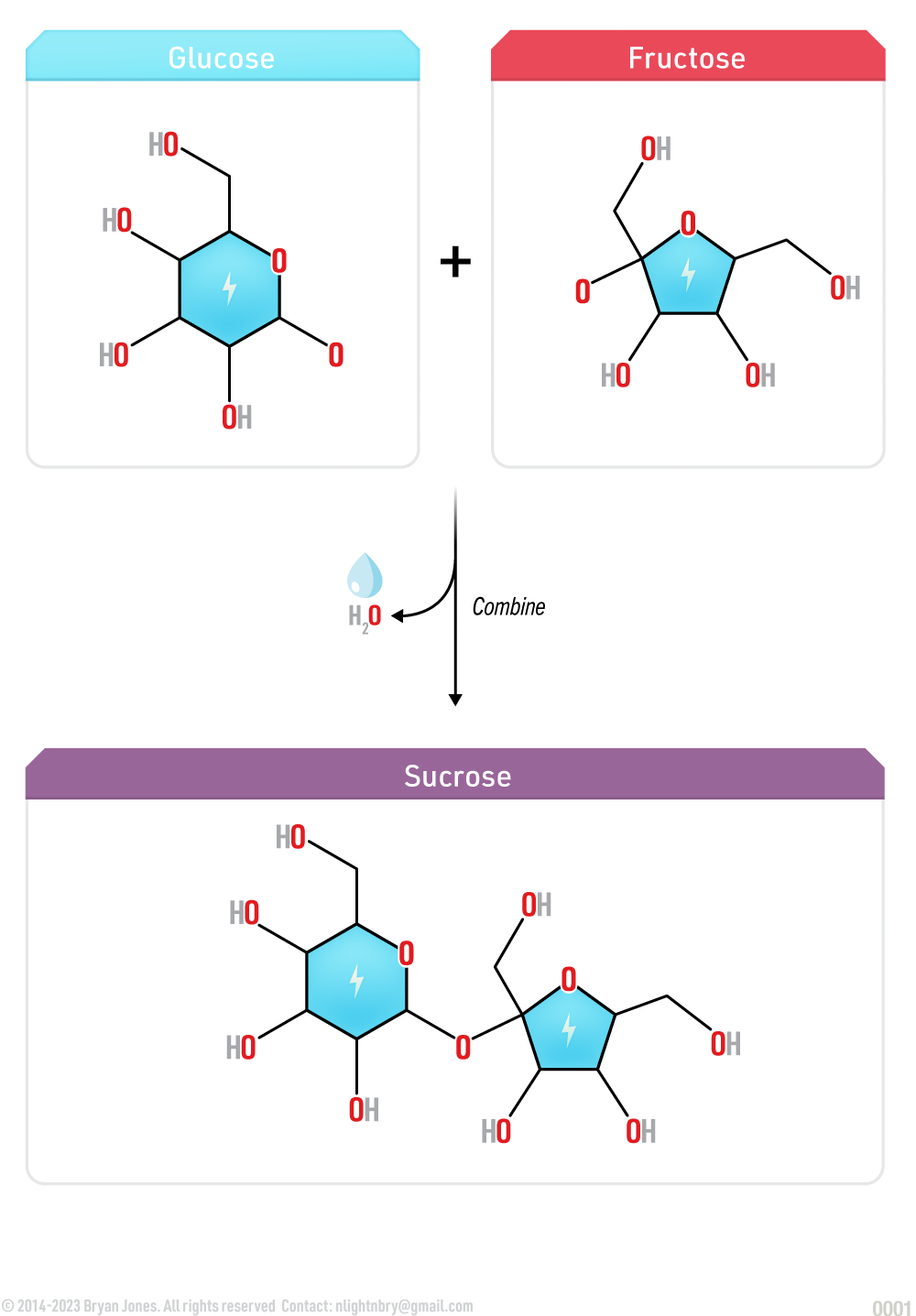
Disaccharides are formed when two monosaccharides bond in dehydration synthesis reaction. Plants utilize the metabolic pathway known as photosynthesis to produce sucrose, an example of a disaccharide comprised of glucose and fructose. A disaccharide can be hydrolyzed to produce the two seperated monosaccharides.
Generalized Disaccharide

Disaccharide (Sucrose)


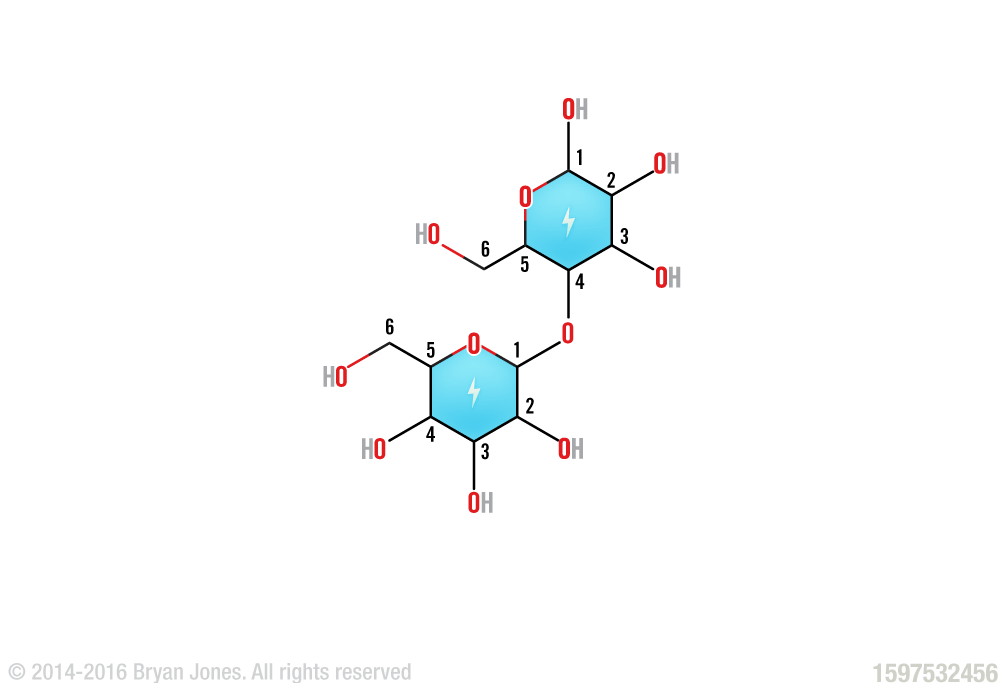
Disaccharides formed from two monosaccharides are the simplest polysaccharides. The disaccharide lactose, composed of galactose and glucose, is the major sugar in milk.
Lactose (Galactose-Glucose)
The disaccharide sucrose, composed of glucose and fructose, is a principal product of plant photosynthesis and is refined into common table sugar.
Your ass is amazing, a perfect 10 because you know about High-fructose corn syrup (HFCS) and exercise a lot. Telling your partner to pound it might break a few Fructose bonds, but it's more likely going to cause them to burn all of theirs.
High-fructose corn syrup (HFCS) which is a mixture of fructose and glucose may take longer for the body to break down compared to other forms of sugar due to its composition and the way it is metabolized. The metabolism of fructose involves several enzymatic reactions, These intermediates can enter the glycolytic pathway and be converted into glucose or stored as fat.
Fructose is metabolized in the liver, while glucose is metabolized all over the body. The metabolism of fructose in the liver can lead to the production of fatty acids (FAT), triglycerides (FAT), and uric acid, which can contribute to adverse health effects when consumed in excess. This metabolic process may be one reason why high intakes of fructose, including from HFCS, have been associated with metabolic disorders such as obesity, insulin resistance, and non-alcoholic fatty liver disease.
Excess amounts of glucose can also provide energy for bacteria to grow which can cause your skin to breakout, this is why your body has a mechanism to keep your blood glucose levels at a healthy level. Controlling glucose prevents unhealthy organisms from growing and your immune system targets them and destroying them.
Sucrose (Glucose-Fructose)
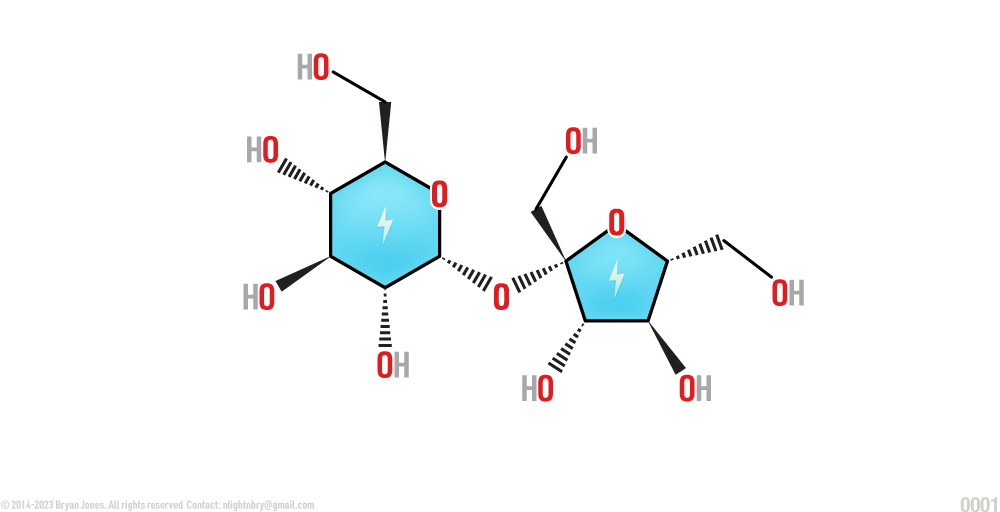
Polysaccharides
Polysaccharides are composed of long chains of monosaccharides and function as storage, structure, and protection.
The most common storage carbohydrate in animal cells is glycogen, a very long, highly branched polymer of glucose units. 10 percent of our livers weight can be glycogen.
Within the Human digestive system, enzymes can hydrolyze the glycosidic bonds in starch, but not the glycosidic bonds in cellulose. Many species of plants, bacteria, and molds produce cellulose-degrading enzymes. Cows also contain these enzymes.
Polysaccharide (Glycogen)
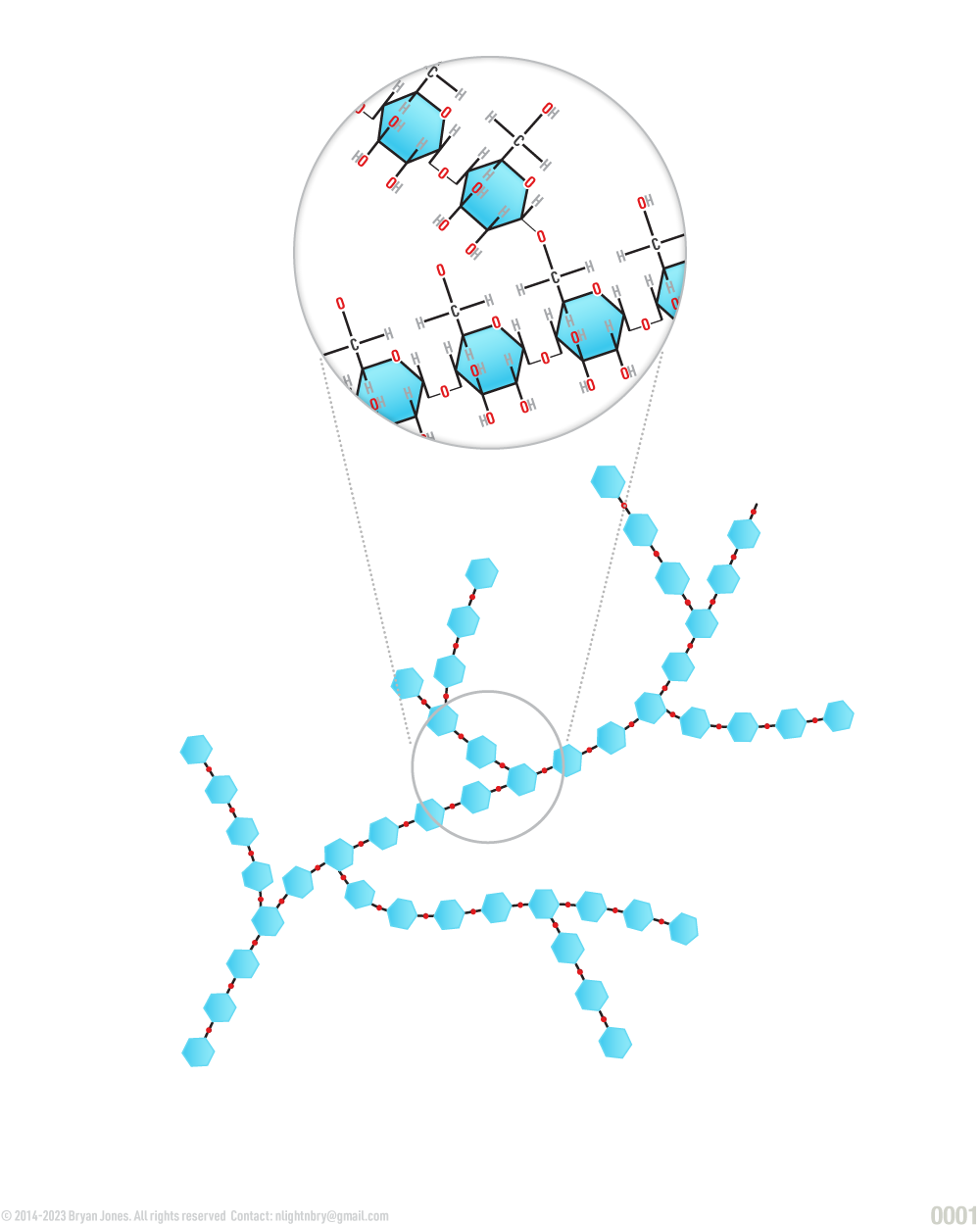
Cellulose, the major constituent of plant cell walls, is an unbranched polymer of the anomer of glucose. Starch, the glucose polymer (disaccharide) is used as primary storage carbohydrate for plant cells. Starch occurs in an unbranched form amylose and lightly branched form amylopectin.
Polysaccharide (Cellulose)

Glycosidic Bond Formation
Glycosidic bond formation is a dehydration reaction which results in the loss of a water molecule from the reactants. For example, the monosaccharides glucose and fructose which combine to form a molecule of the disaccharide sucrose releases a molecule of water in a hydrolysis reaction.
Glycosidic bond formation is the process of linking two monosaccharides together by a covalent bond. It involves the dehydration synthesis of an anomeric carbon of one sugar with a hydroxyl group of another sugar, resulting in the formation of an acetal or ketal linkage.
The formation of a glycosidic bond is a crucial step in the biosynthesis of polysaccharides, oligosaccharides, and glycoproteins. This process is catalyzed by glycosyltransferases, which transfer the sugar moiety from a nucleotide sugar donor to the hydroxyl group of the acceptor sugar.
There are several factors that influence the formation of glycosidic bonds, including the stereochemistry of the anomeric carbon, the configuration of the hydroxyl group on the acceptor sugar, and the presence of neighboring functional groups.
Overall, glycosidic bond formation plays an essential role in the structure and function of carbohydrates, which are involved in a wide range of biological processes, including energy storage, cell signaling, and cell-cell recognition.
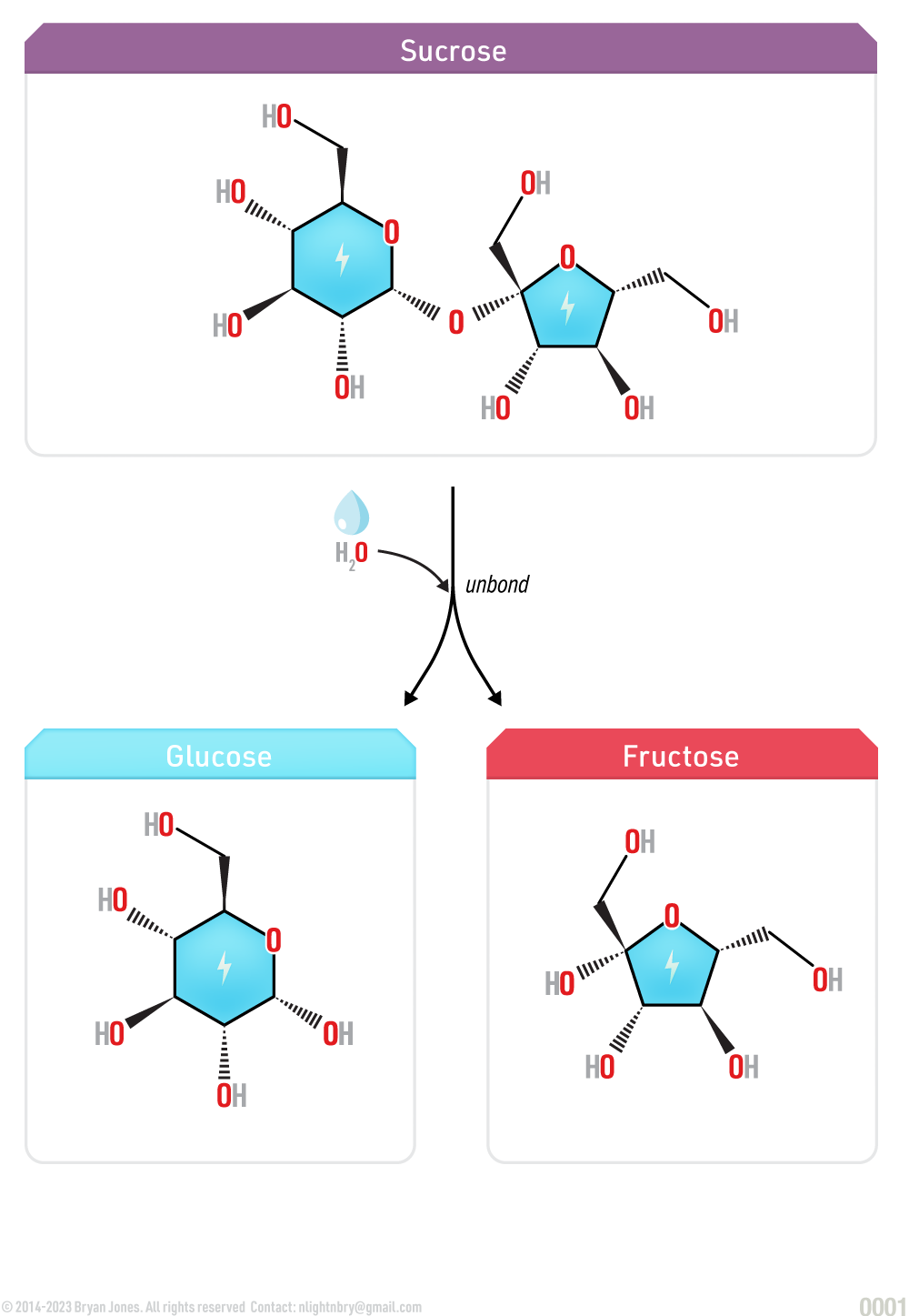
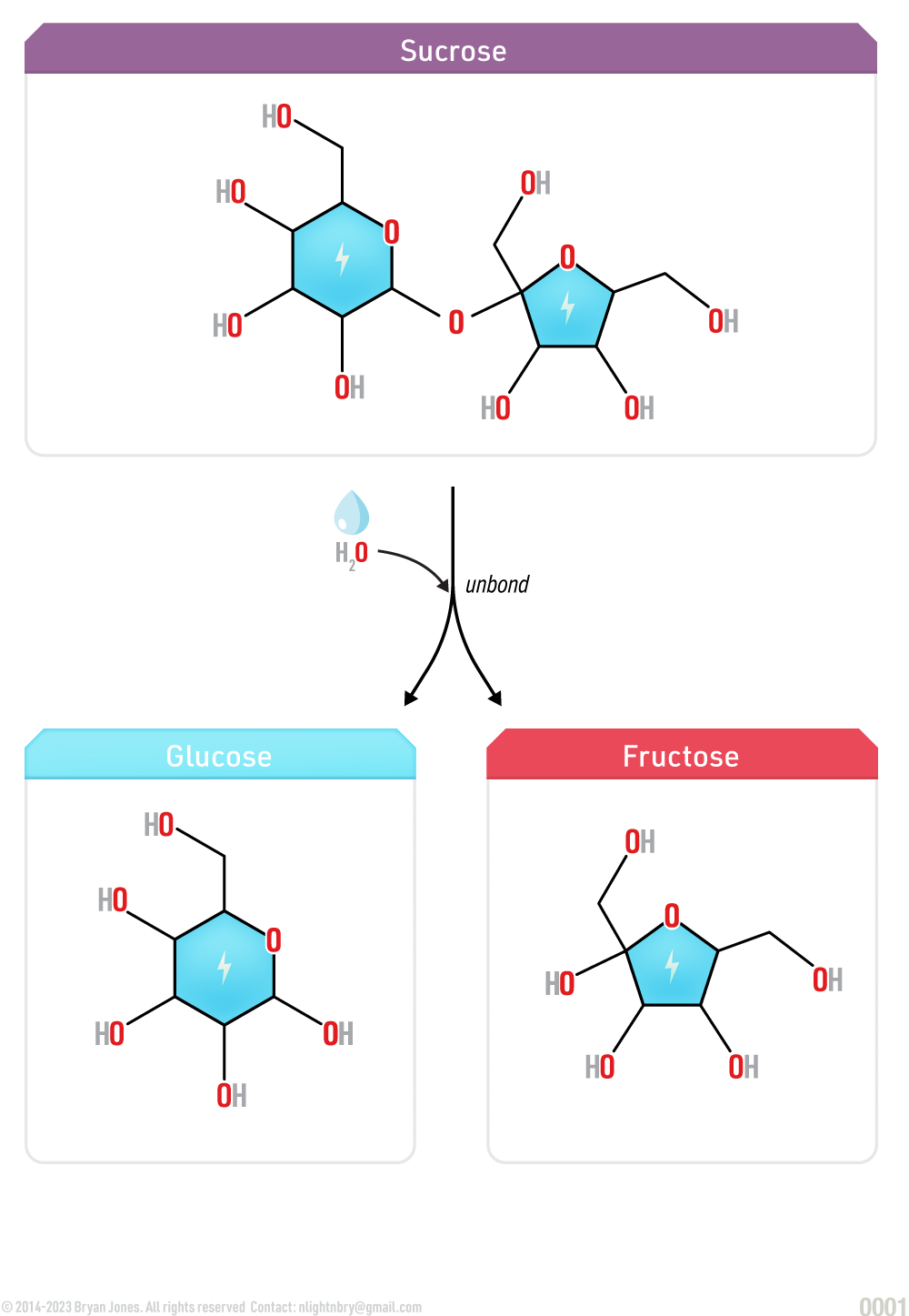
Hydrolysis Reaction
Hydrolysis is the breakdown of polysaccharides into simple carbohydrates with water.
When a chemical compound reacts with water, this chemical reaction is known as hydrolysis. Hydrolysis is used to break down polymers. For example, when sucrose molecule breaks down into smaller molecules glucose and fructose hydrolysis occurs.
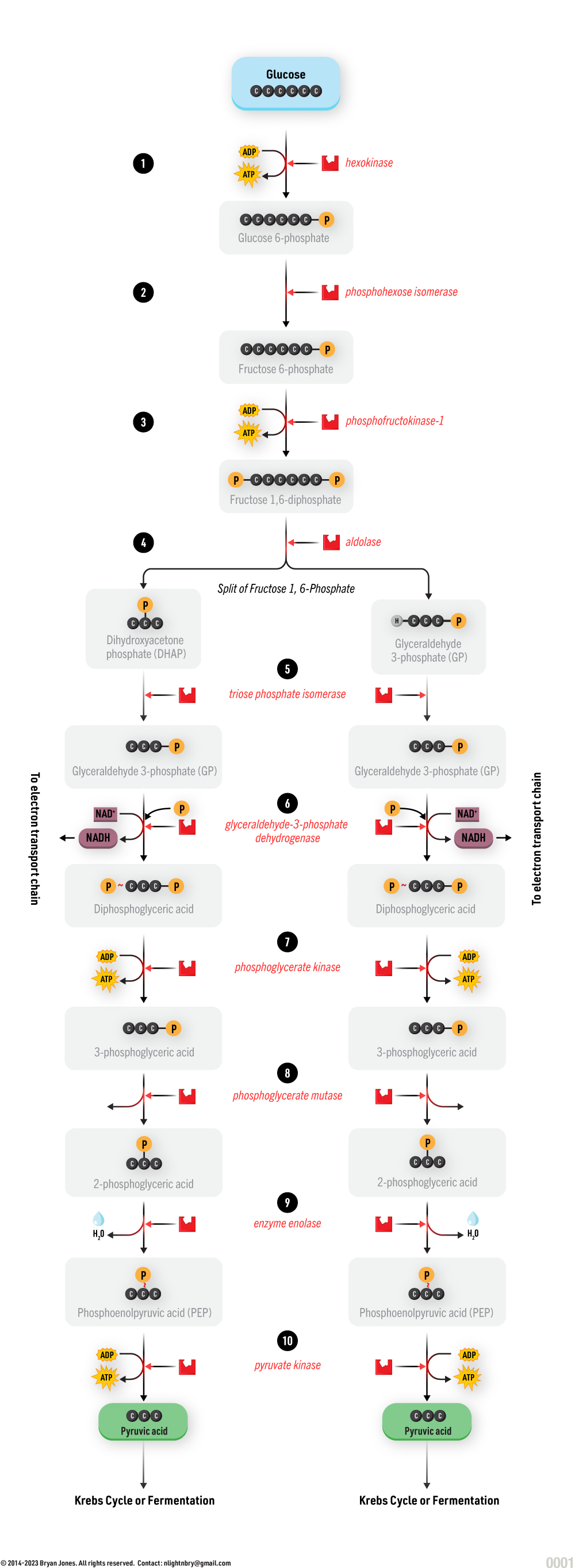
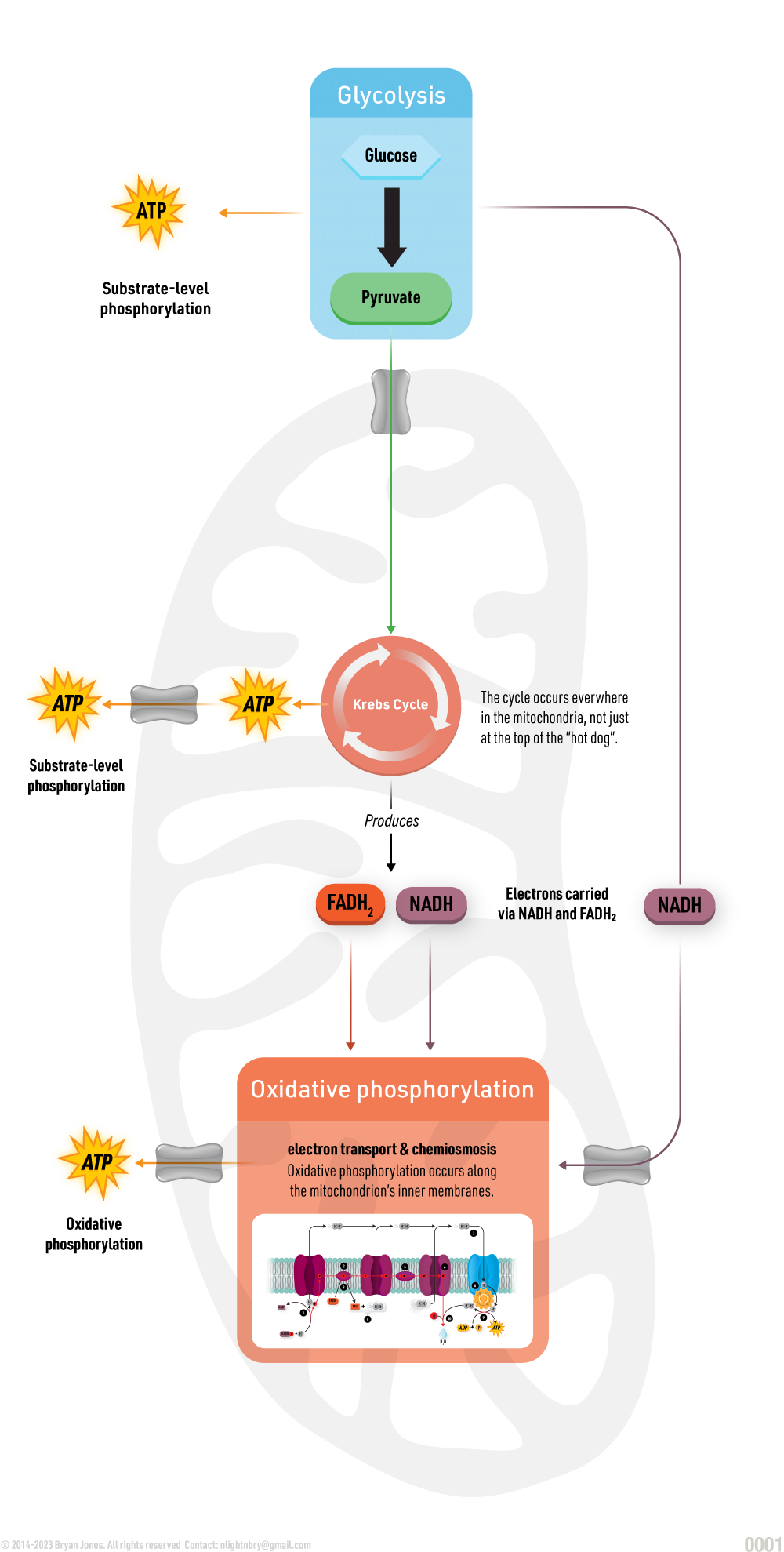
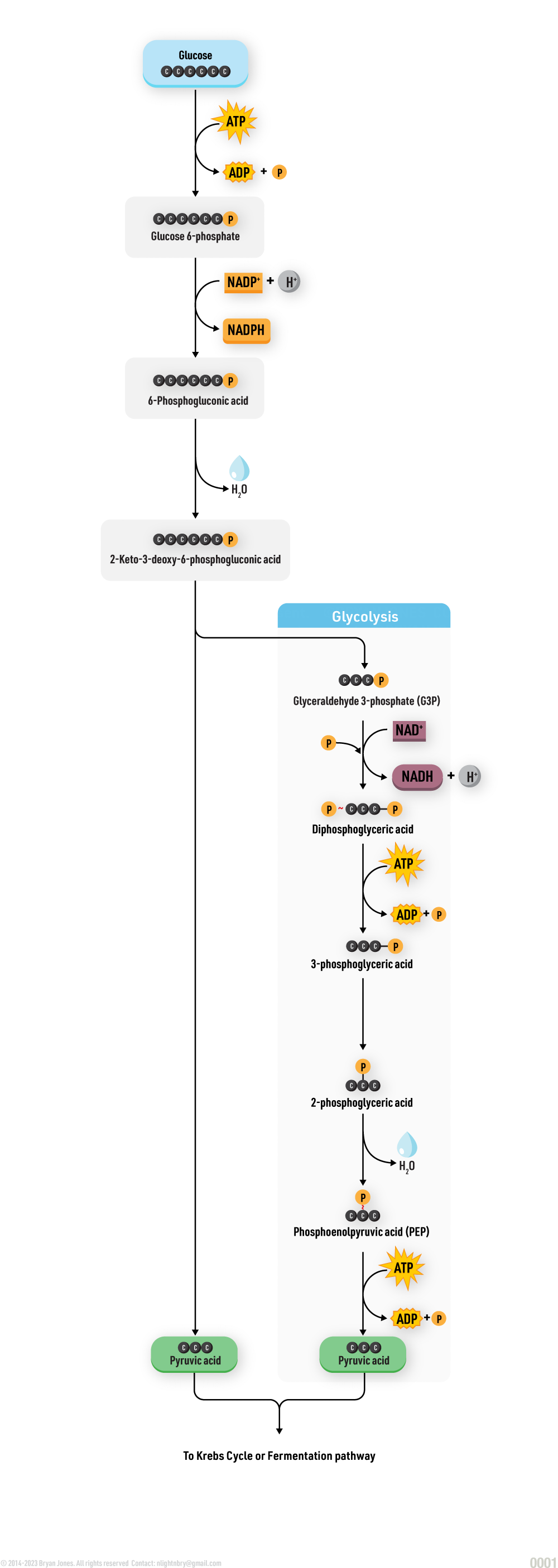
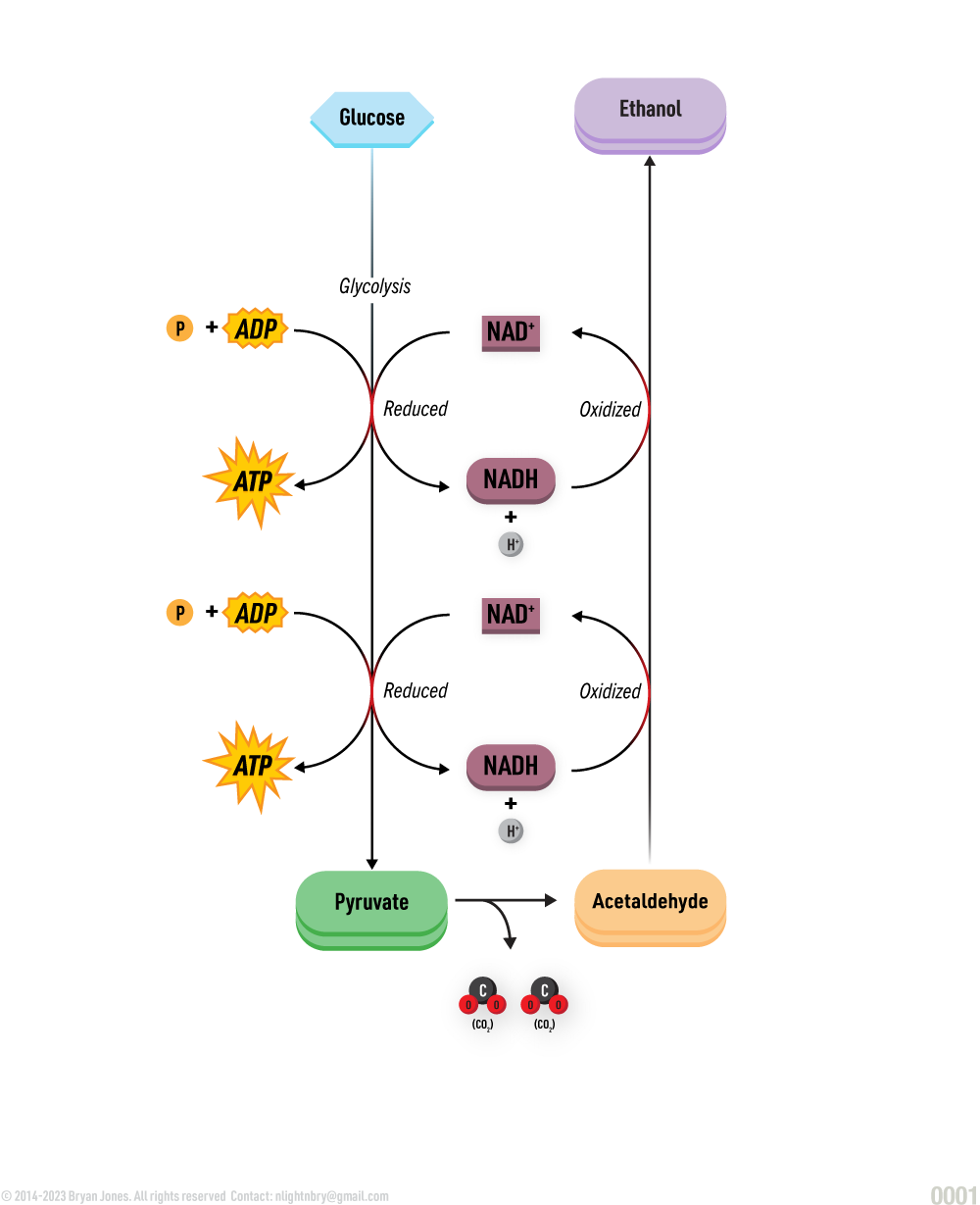
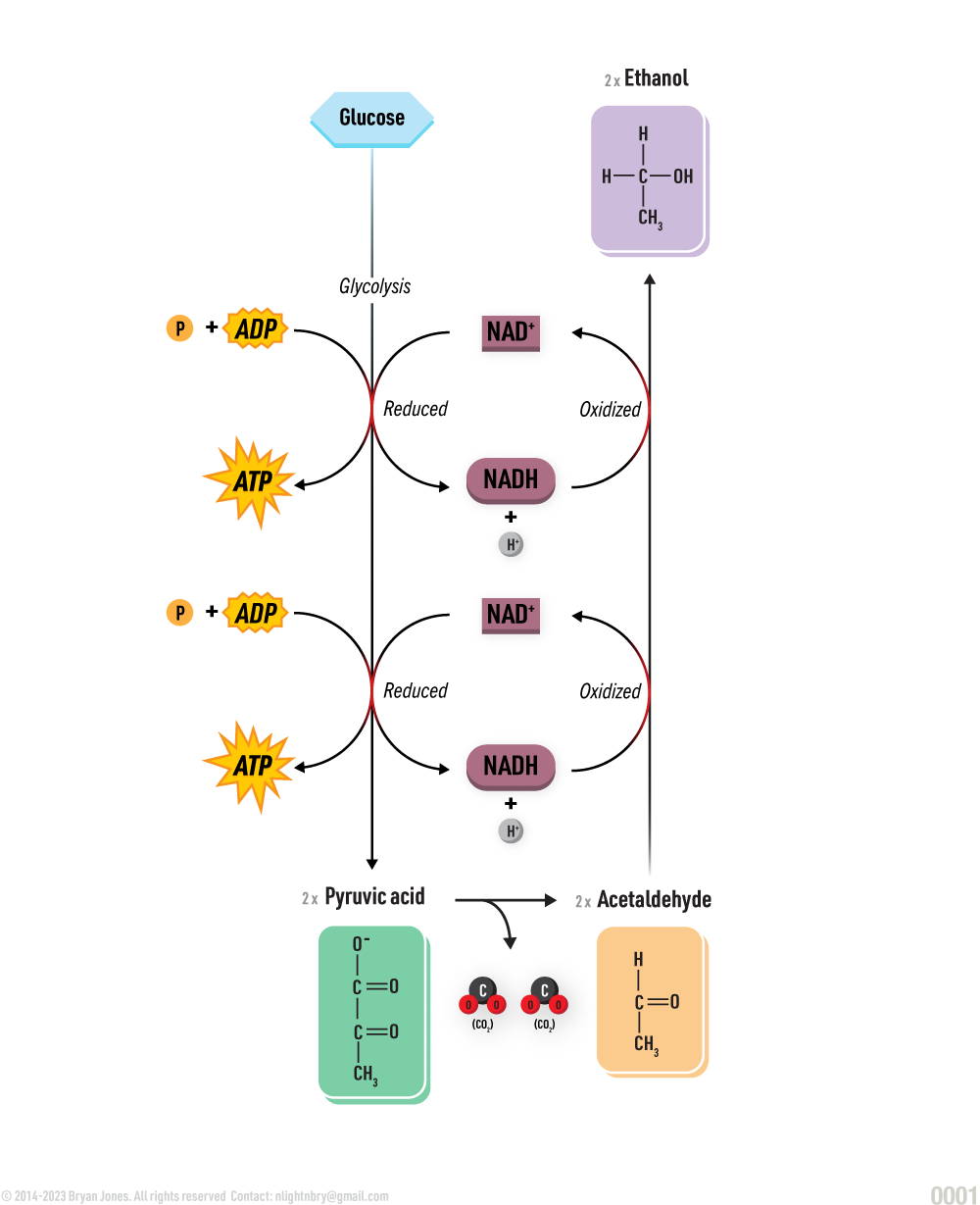
Carbohydrate Catabolism
Glycolysis, Cellular Respiration, Entner-Doudoroff, and Fermentation
Catabolism will be explained in detail within other sections. Here are four pathways that breakdown Glucose for energy and as structure to build on.
Carbohydrates Summary
Carbohydrates play several important roles in the body, including providing energy for cellular metabolism, serving as a structural component of cell membranes, and playing a role in cell-cell recognition and signaling. They also serve as precursors for the synthesis of other biomolecules, such as nucleotides and amino acids.
Carbohydrates Advanced Summary
Basic Carbohydrates chapter for a medical student:
*This is not an exhaustive list but rather a sample of the many topics that could be included in a comprehensive carbohydrates chapter for a medical student.