The Atom
The atom is the basic unit of matter consisting of a nucleus at the center which is composed of neutrons and protons, and a surrounding cloud of negatively charged electrons.
The nucleus has a consistent mass, and that mass is how we differentiate elements from one another.
The Structure of an Atom:
Protons, neutrons, electrons
An atom consists of a nucleus at the center, composed of protons (positively charged particles) and neutrons (uncharged particles). Surrounding the nucleus, electrons (negatively charged particles) move more freely, in layers known as electron shells. The electron shells do not represent orbits; instead, they represent energy levels or regions of space in which an electron can live. The electrons on the innermost circle closest to the nucleus have the lowest energy.
The Structure of an Atom:
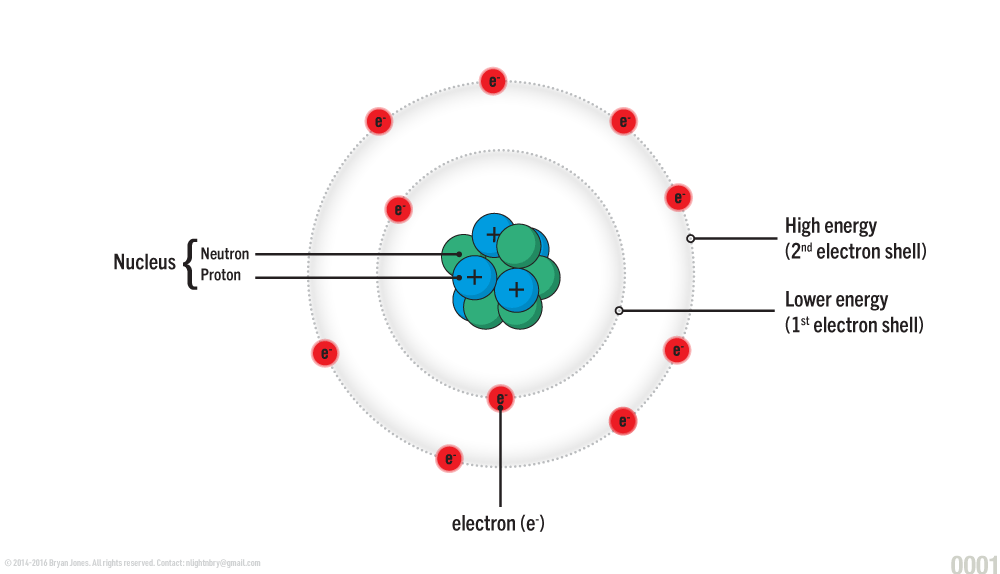
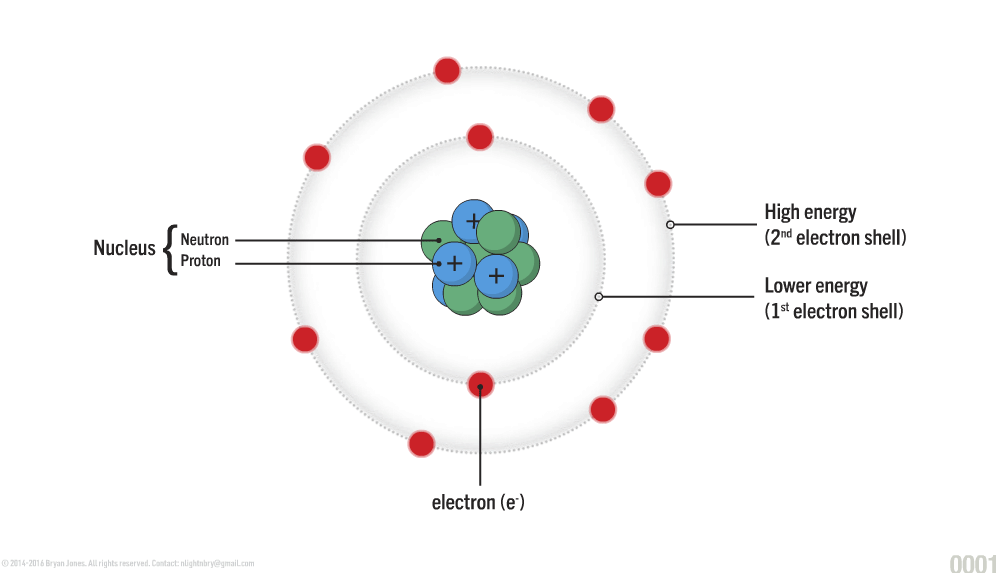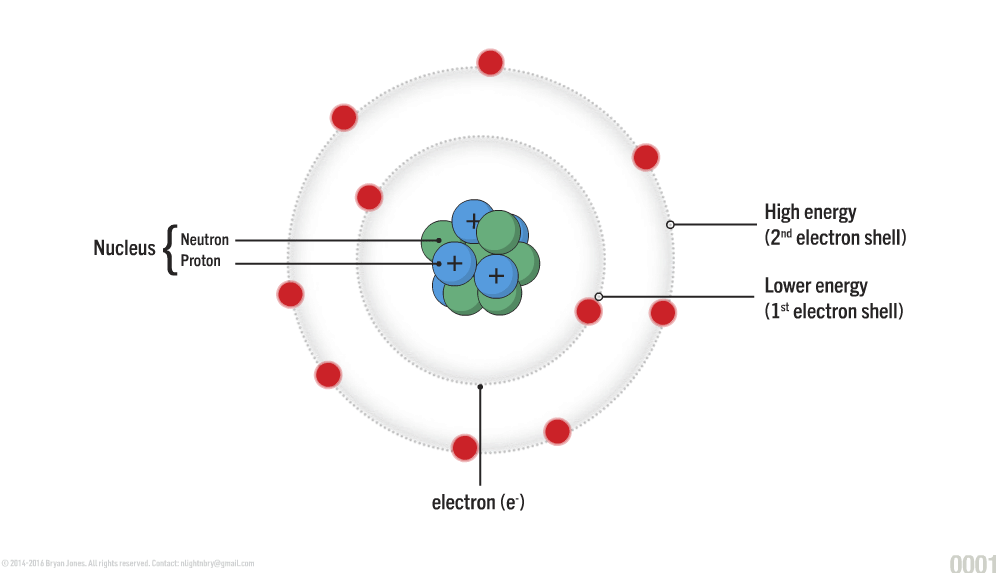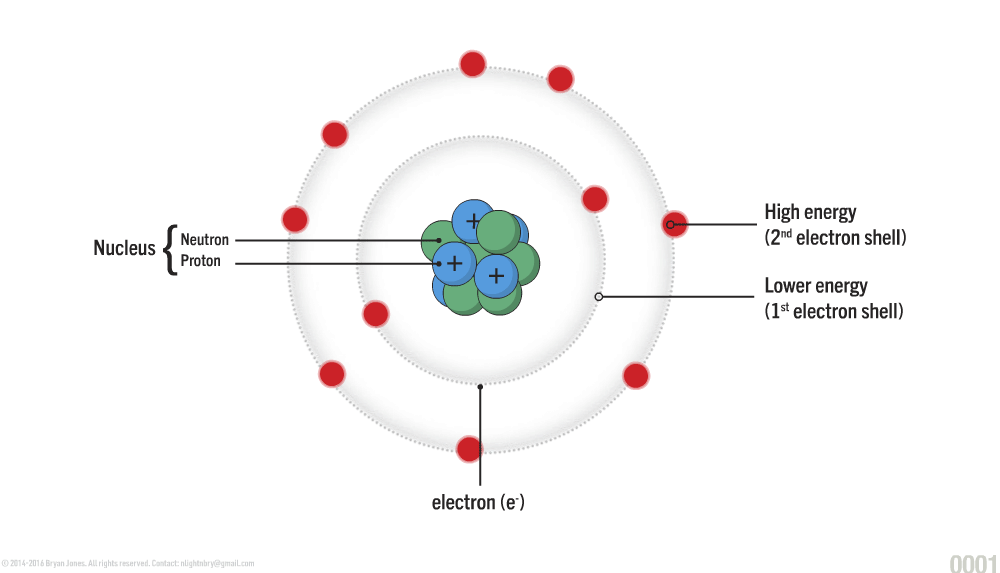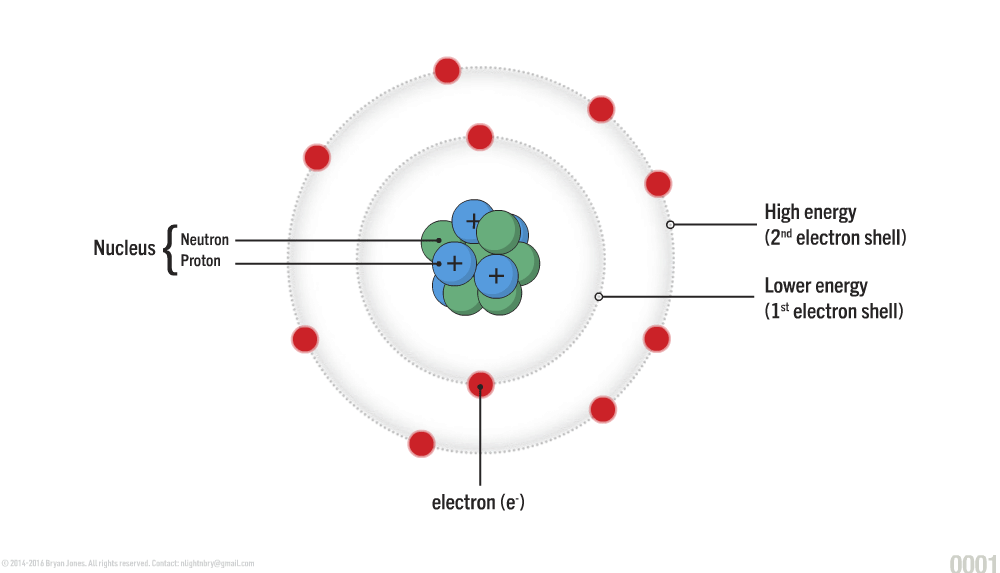
Electron
Electrons are the lightest particle in the atom and occupy the cloud or shell, of the atom. They carry a negative charge.
- Electrons can collide with certain particles, becoming diffracted as light. This means an electron has wave-like and particle-like characteristics.
- Electrons create negatively charged electric fields which are attracted to other atoms’ positively charged particles (protons).
- Electrons also create magnetic fields, through mass motion.
- An atomic orbital is the function of a bound electron behaving in a wave-like manner.
- Orbital transfer occurs when photons are emitted or absorbed, causing electrons to transfer from one orbital to another.
- Electromagnetic interactions result in the occurrence of chemical bonds between atoms.
- Electrons determine whether a particle has a positive or negative charge, depending upon the balance between the number of electrons and the positively charged nuclei. If an atom has more negative electrons than protons, it is negatively charged or vice versa.
Proton
The proton is the heaviest of the subatomic particle (neutrons, electrons). Located in the nucleus, or center, of the atom, its carries a positive charge.
- When an atom is in a stable state, the number of protons and electrons are equal.
- Protons are an elementary particle that can exist by themselves, however, with the goal of balance, they combine with neutrons which then pick up electrons. This completes the creation of an atom.
- The atomic number of an atom is the number of protons in an atom.
- Protons preside in the nucleus of an atom
Neutron
The neutron is similar to the proton in its location - at the core of the atom, or nucleus. It is also heavy - though not as heavy as the proton. It differs in that it carries a neutral charge - neither positive nor negative.
- Neutrons are not electrically charged.
- Neutrons help establish the mass of an atom.
- It is the mass of the atom which defines it as a discrete element.
The diameter of a helium nucleus is about 100,000 times smaller than the diameter of a helium atom. This means that if you could see a helium nucleus, it would appear as a tiny point compared to its electron cloud.
Helium Atom Size
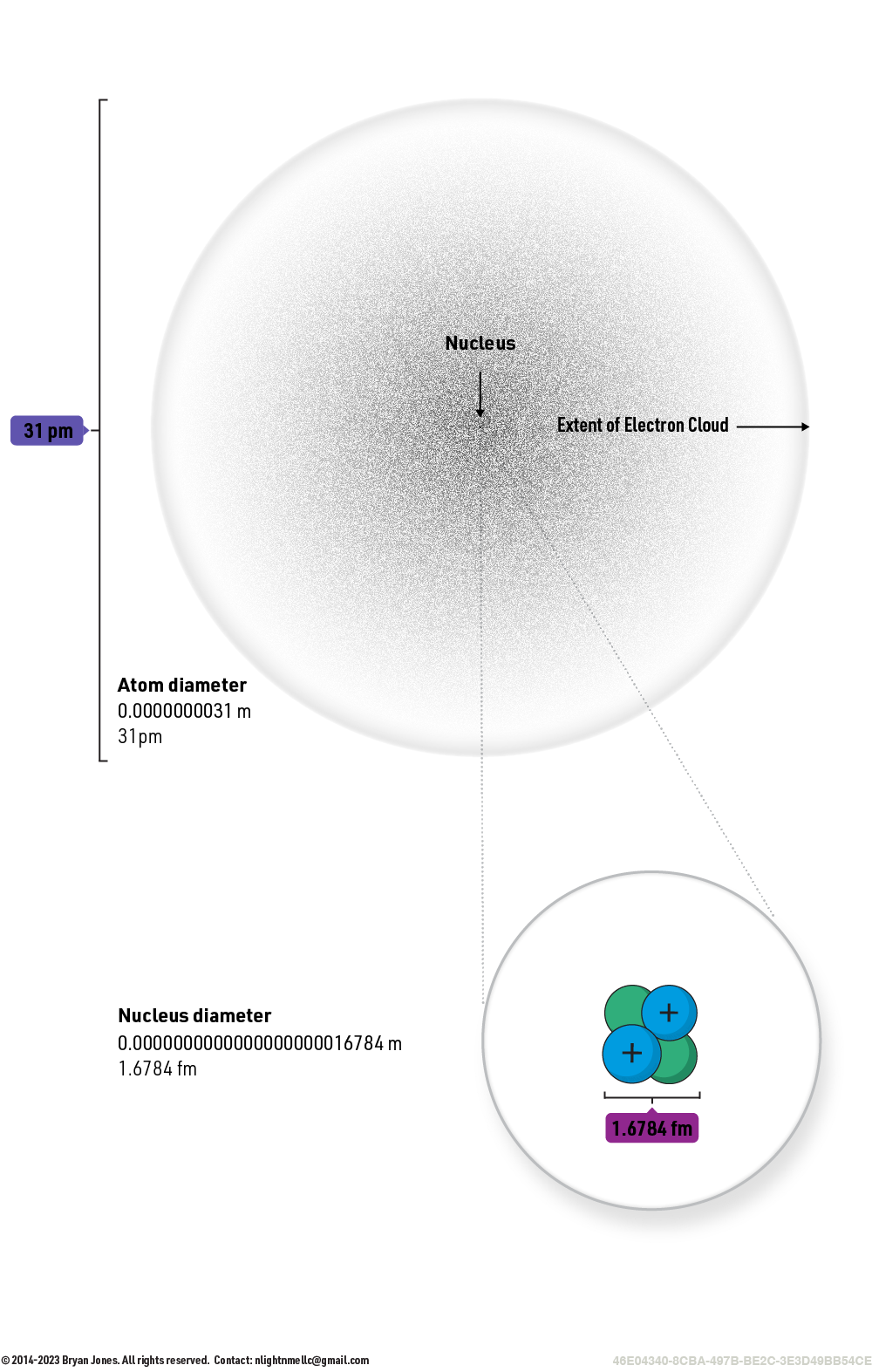
The diameter of a helium nucleus is about 1.67824 fm (femtometers). A femtometer is a unit of length equal to 10^-15 m. It is a very small unit, and it is used to measure the size of atomic nuclei.
Metric System
| Prefix | Symbol | Power of Ten |
|---|---|---|
| Yotta- | Y | 1024 |
| Zetta- | Z | 1021 |
| Exa- | E | 1018 |
| Peta- | P | 1015 |
| Tera- | T | 1012 |
| Giga- | G | 109 |
| Mega- | M | 106 |
| Kilo- | k | 103 |
| Hecto- | h | 102 |
| Deca- | da | 101 |
| Deci- | d | 10-1 |
| Centi- | c | 10-2 |
| Milli- | m | 10-3 |
| Micro- | µ | 10-6 |
| Nano- | n | 10-9 |
| Pico- | p | 10-12 |
| Femto- | f | 10-15 |
| Atto- | a | 10-18 |
| Zepto- | z | 10-21 |
| Yocto- | y | 10-24 |
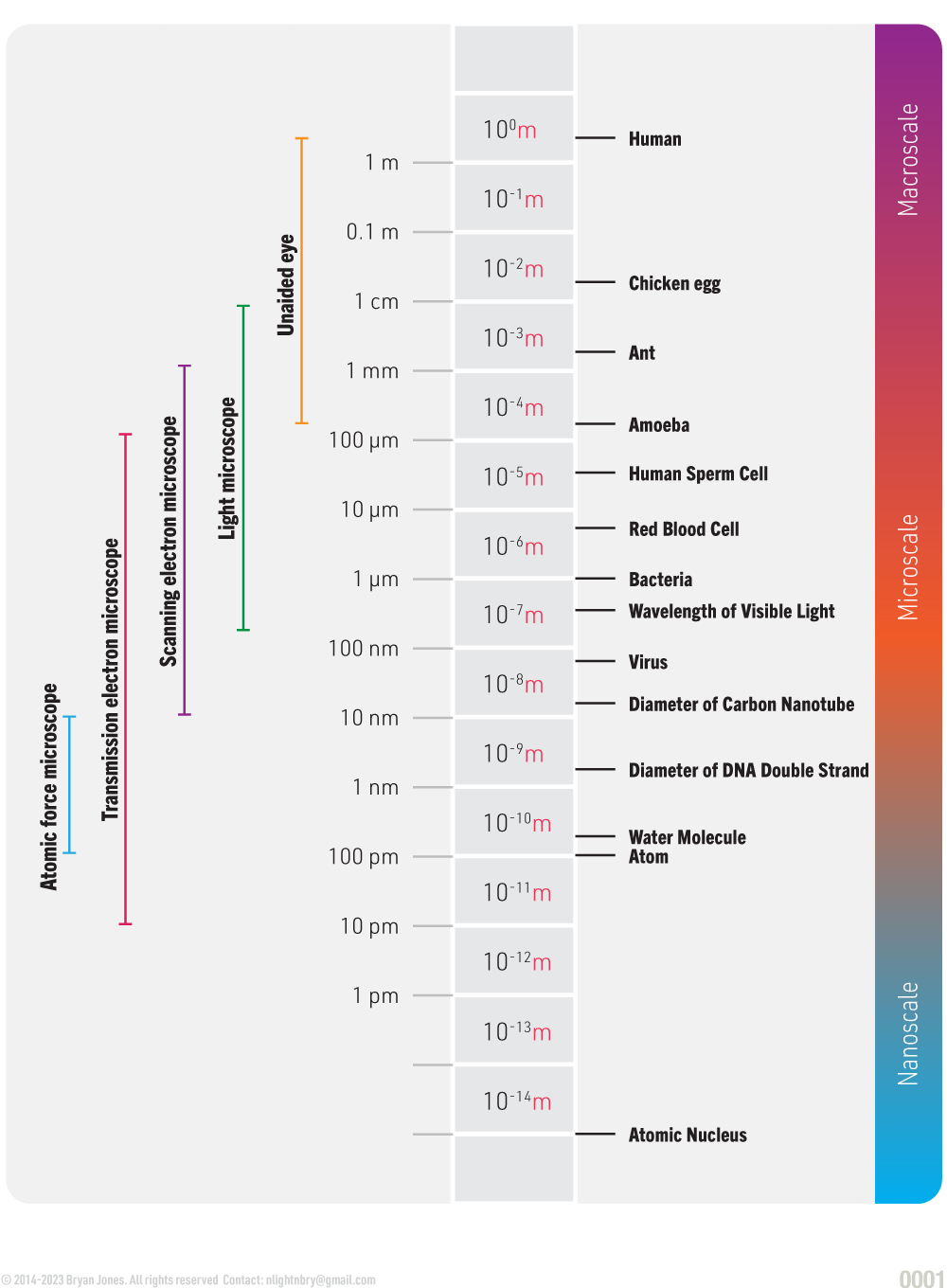
Scale
You'll need to consider scale, it's not important at the beginning but later will help you.
Proton
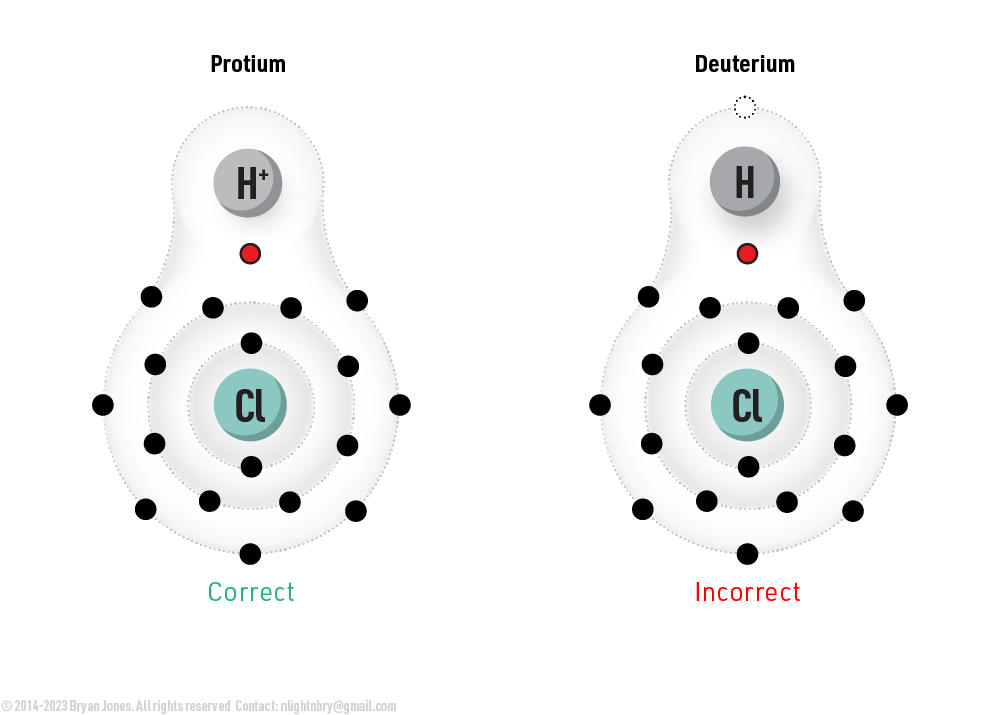
Protons, Protium and why it's called Hydrogen
The chemical symbol for hydrogen is H. When hydrogen has a positive charge, it is written as H+. The plus sign indicates that the hydrogen atom has lost an electron, leaving it with one proton and one electron. This is called a protonated hydrogen atom.
Its Protium when you see a single Electron-Proton pair or H+
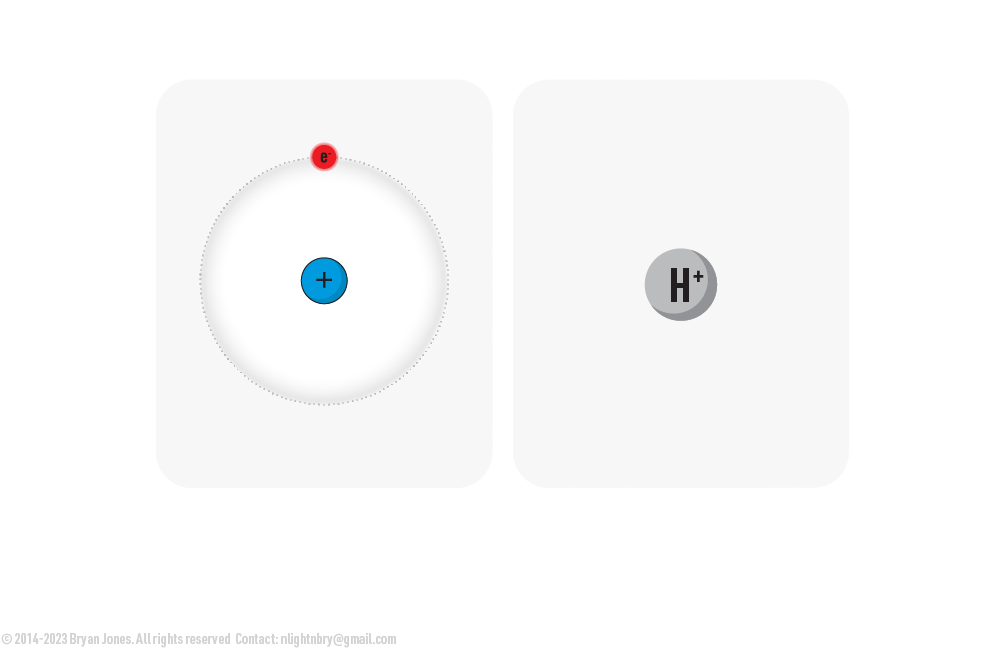
When a molecule has a positive charge, it means that it has more protons than electrons. This can happen in two ways:
- The molecule can have more protons than it normally does. This can happen if the molecule gains a proton, or if it loses an electron.
- The molecule can have the same number of protons as it normally does, but it can lose one or more electrons. This can happen if the molecule is in an environment with a lot of electrons, such as a solution of water.
In both cases, the molecule will have a net positive charge. When this happens, the protons in the molecule are often referred to as "hydrogens". This is because the protons are the only part of the molecule that has a positive charge.
For example, the molecule of hydrochloric acid (HCl) has a positive charge. This is because it has one proton and one electron.
This is just a convention, and it is not always strictly accurate. However, it is a convenient way to refer to the positive charge in a molecule. Just consider that hydrogen has there isotopes, of which protium has the greatest abundance.
Hydrochloric acid (HCl) Molecule

When a molecule gains a proton, where is the proton coming from?
When a molecule gains a proton, the proton can come from a number of sources. One possibility is that the proton comes from another molecule. This can happen if two molecules react with each other, and one molecule donates a proton to the other molecule.
Another possibility is that the proton comes from the environment. This can happen if the molecule is in a solution of water, for example. The water molecules can donate protons to the molecule, which will give the molecule a positive charge.
Finally, the proton can also come from the breakdown of a larger molecule. For example, if a molecule of glucose is broken down into two molecules of pyruvate, one of the pyruvate molecules will have a positive charge because it will have gained a proton.
The exact source of the proton will depend on the specific reaction or process that is taking place. However, in all cases, the proton will come from another molecule, the environment, or the breakdown of a larger molecule.
HCl Molecule within H2O Solution
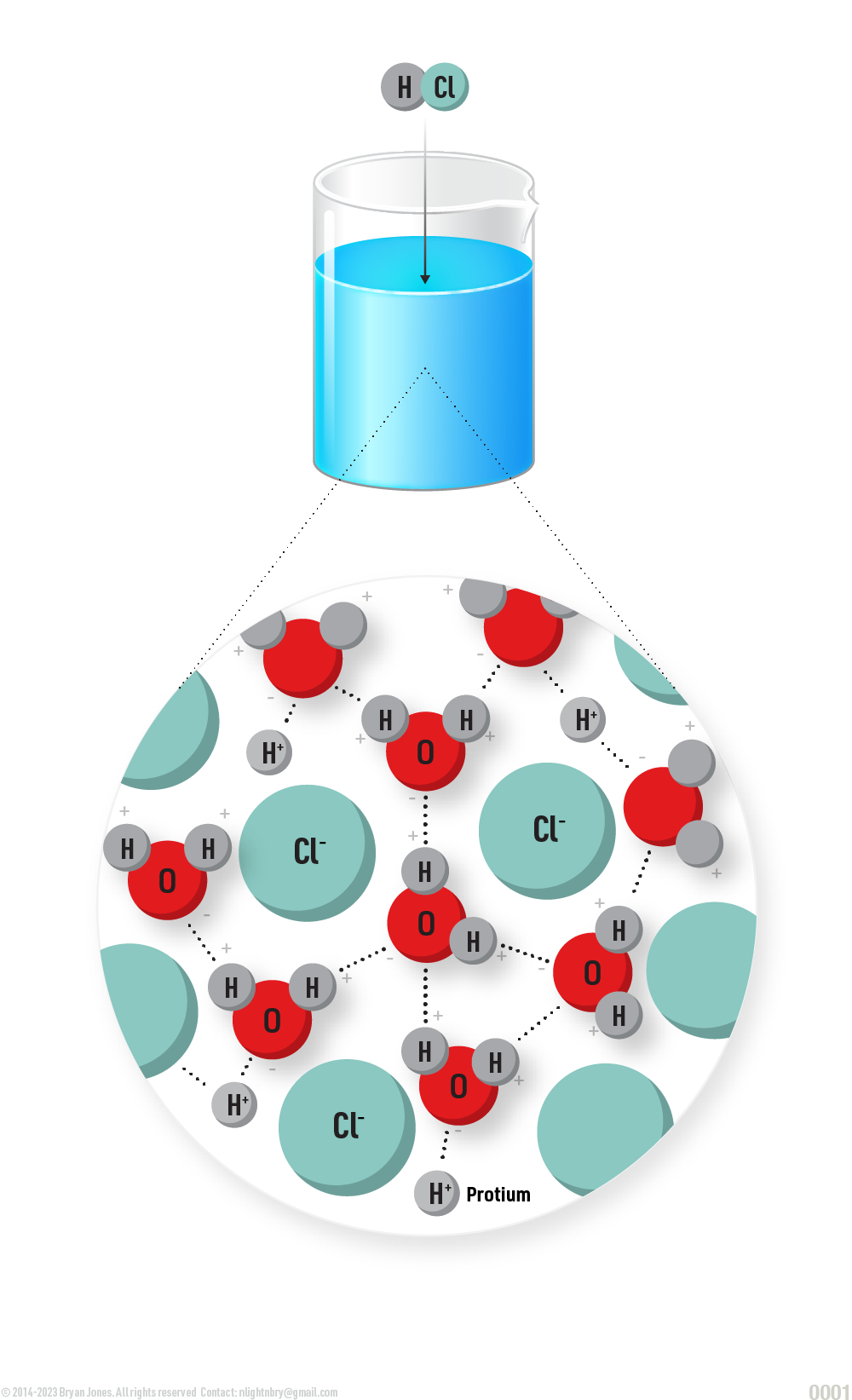
Here are some examples of reactions where a molecule gains a proton:
- The reaction of hydrochloric acid (HCl) with water:
HCl + H2O → H2O+ + Cl- - The reaction of ammonia (NH3) with water:
NH3 + H2O → NH4+ + OH- - The breakdown of glucose into pyruvate:
C6H12O6 → 2 CH3COCOO- + 2 H+
In all of these reactions, the molecule that gains a proton has a positive charge. This is because the proton has a positive charge, and when it is added to the molecule, it gives the molecule a net positive charge.
A single proton in solution is just a protium?
Yes, a single proton in solution is just a protium. Protium is the most common isotope of hydrogen, and it is made up of a single proton and no neutrons. The proton is the positively charged particle that is found in the nucleus of an atom, and it is what gives the atom its identity.
When a proton is in solution, it is surrounded by water molecules. The water molecules interact with the proton and help to stabilize it. The proton is also able to interact with other protons in solution, and this can lead to the formation of hydrogen bonds.
Protium is a very stable isotope, and it is not radioactive. It is also the most abundant isotope of hydrogen, making up about 99.985% of all hydrogen atoms in the universe.
So, if you have a single proton in solution, you can be sure that it is just a protium.
Proton
Protons, Protium and why it's called Hydrogen
- When an atom is in a stable state, the number of protons and electrons are equal.
- Protons are an elementary particle that can exist by themselves, however, with the goal of balance, they combine with neutrons which then pick up electrons. This completes the creation of an atom.
- The atomic number of an atom is the number of protons in an atom.
- Protons preside in the nucleus of an atom
- The molecule can have more protons than it normally does. This can happen if the molecule gains a proton, or if it loses an electron.
- The molecule can have the same number of protons as it normally does, but it can lose one or more electrons. This can happen if the molecule is in an environment with a lot of electrons, such as a solution of water.
Isotopes Affect Electrons
Isotopes are an atomic species in which an element has variation in mass.
Electrons have a trajectory, or at least this helps our brains understand their movements. Let's use a hydrogen atom as an example. Hydrogen has one electron moving around a proton and neutron, which form an electric field that affects the movement of its electron.
Hydrogen:
Hydrogen has three isotopes: protium (1H), deuterium (2H), and tritium (3H). Each isotope has the same proton count, but the amount of neutrons change. Neutrons have a neutral change and affects the mass of the nucleus, which affects the electron movement gravitationally. Neutrons basically force the electrons to move around the extra neutrons.
Hydrogen Isotopes:

Movement around mass
The shift in electron movement is due to the heavier isotope exerting a stronger gravitational force on the electron, causing it to move slightly closer to the nucleus. This results in a slightly shorter average distance between the electron and the nucleus, and a slightly lower energy level for the electron. The analogy of a moon orbiting a planet with mountain ranges on Earth can be used to understand this concept. As the moon travels over the mountains, the extra mass of the mountains causes the moon to move slightly closer to the planet. This is similar to how the heavier isotope causes the electron to move slightly closer to the nucleus.
Elementary particles
Each subatomic particle has even smaller units called elementary particles, one of which is a quark. Electrons, protons, and neutrons each have three quarks that act like a gyroscope. Subatomic particles have different types of gyroscopes, which spin in different ways. Other elementary particles also have an effect on protons, neutrons, and electrons. These elementary particles are known as bosons and leptons, but that's for another time.
Electron spin variation
The gyroscope analogy becomes clearer when witnessing a person using a motor to rapidly rotate a bicycle tire. By holding the spinning tire in their hands while seated on a rotating chair, the individual experiences the combined rotational motion of both themselves and the tire. This interaction results in the centrifugal force exerted by the spinning tire affecting the surrounding matter in their environment.
Gyroscopes are used in clocks to counteract gravity's effect on the accuracy of time. They also counter the waves hitting the side of a ship. A gyroscope is a device with X, Y, and Z axes, each of which spins in a direction. The overall spin of X, Y, and Z balances each other. However, for this to be perfect, you need a nitrogen atom surrounded by a buckyball comprised of carbon atoms.
Now, to continue the gyroscope analogy, you need a "thing" to keep all those gyroscopes in place. This is another elementary particle. As the universe tends to recycle its mechanisms, you can imagine there are X, Y, and Z "things" that keep protons, neutrons, and electrons together like a hockey ring. This hockey ring is kept in place by the Higgs Field. It's all a balancing act.
Elementary particles also overlap with other categories of particles, such as electrons-protons and protons-neutrons. Neutrons and electrons do not overlap, otherwise electrons wouldn't spin around a nucleus. Instead, they would stick to it.
Interactions between Electrons and Protons
Electrons and photons can interact in several ways, depending on the energy of the photons and the properties of the material that the electrons are in. Here are a few ways electrons and photons can interact:
- Absorption: When a photon interacts with an atom or molecule, it can be absorbed, transferring its energy to the electrons in the material. This can cause an electron to move to a higher energy level, or it can cause an electron to be ejected from the material altogether (photoelectric effect).
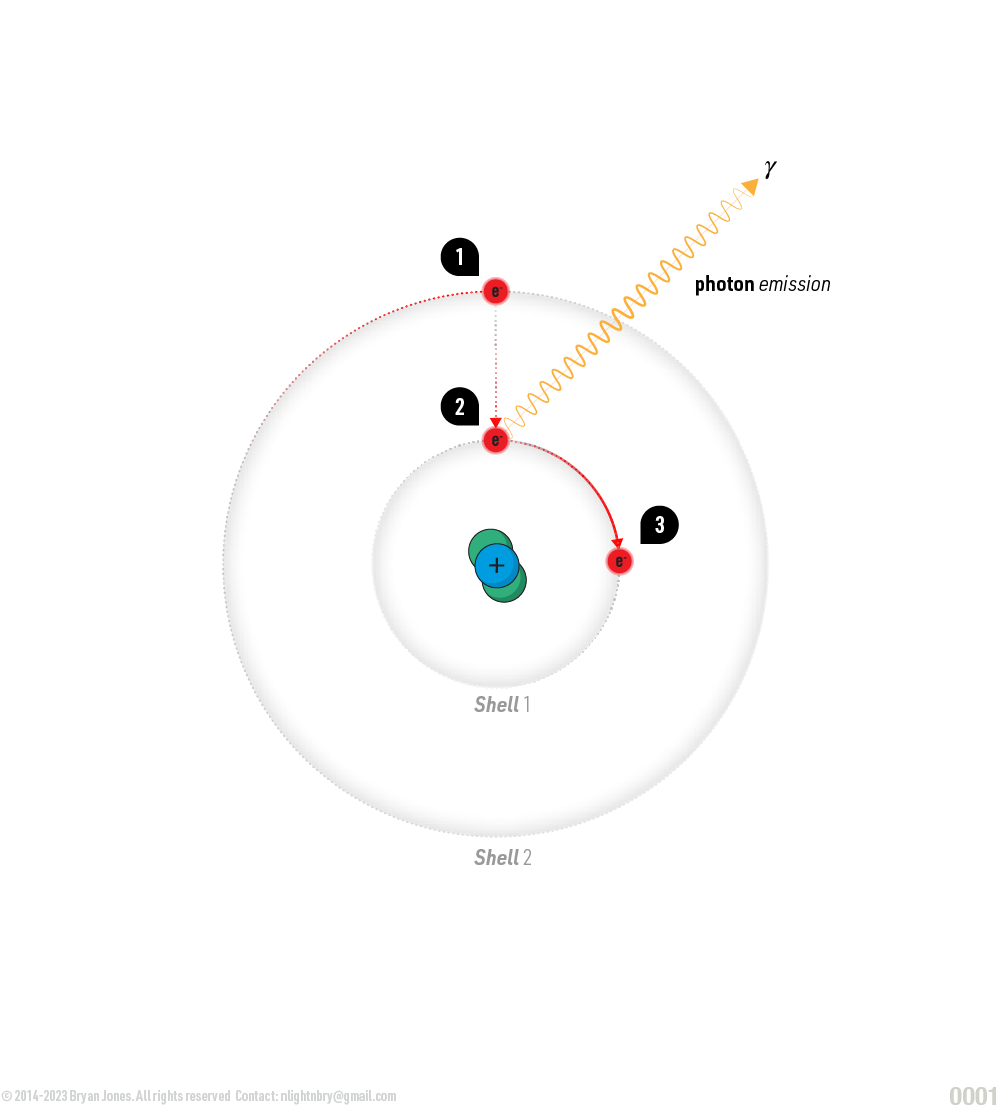
- Emission: Conversely, an excited electron can release energy in the form of a photon, returning to a lower energy state. This process is known as emission or spontaneous emission.
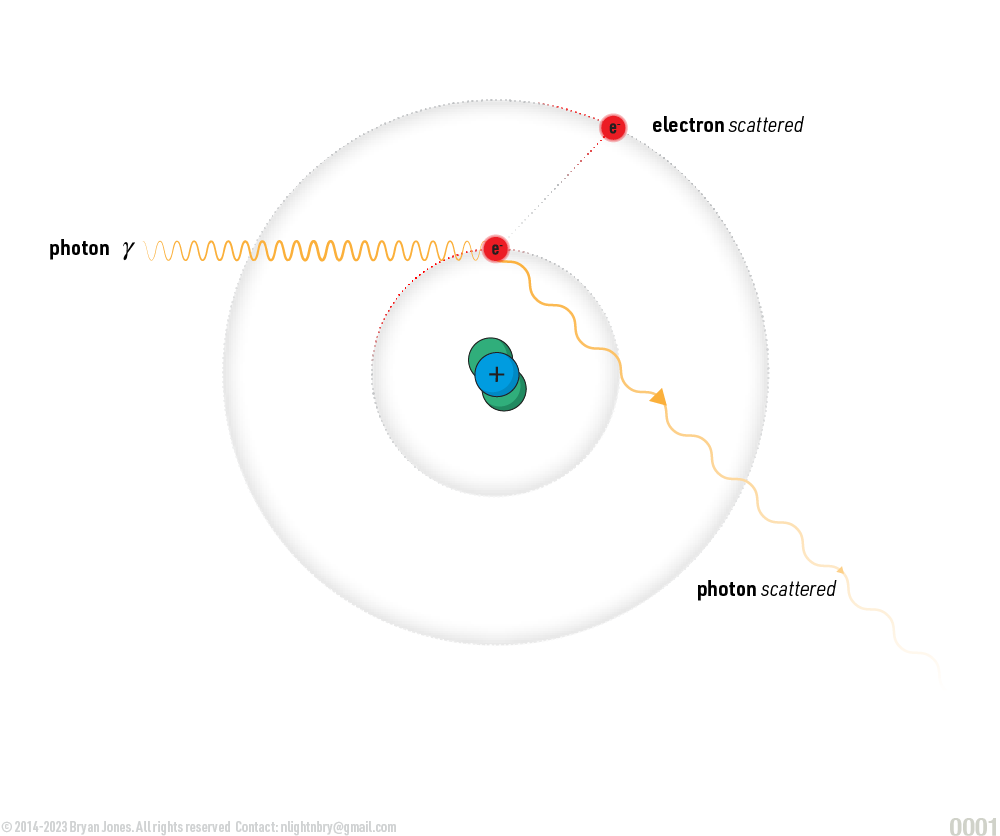
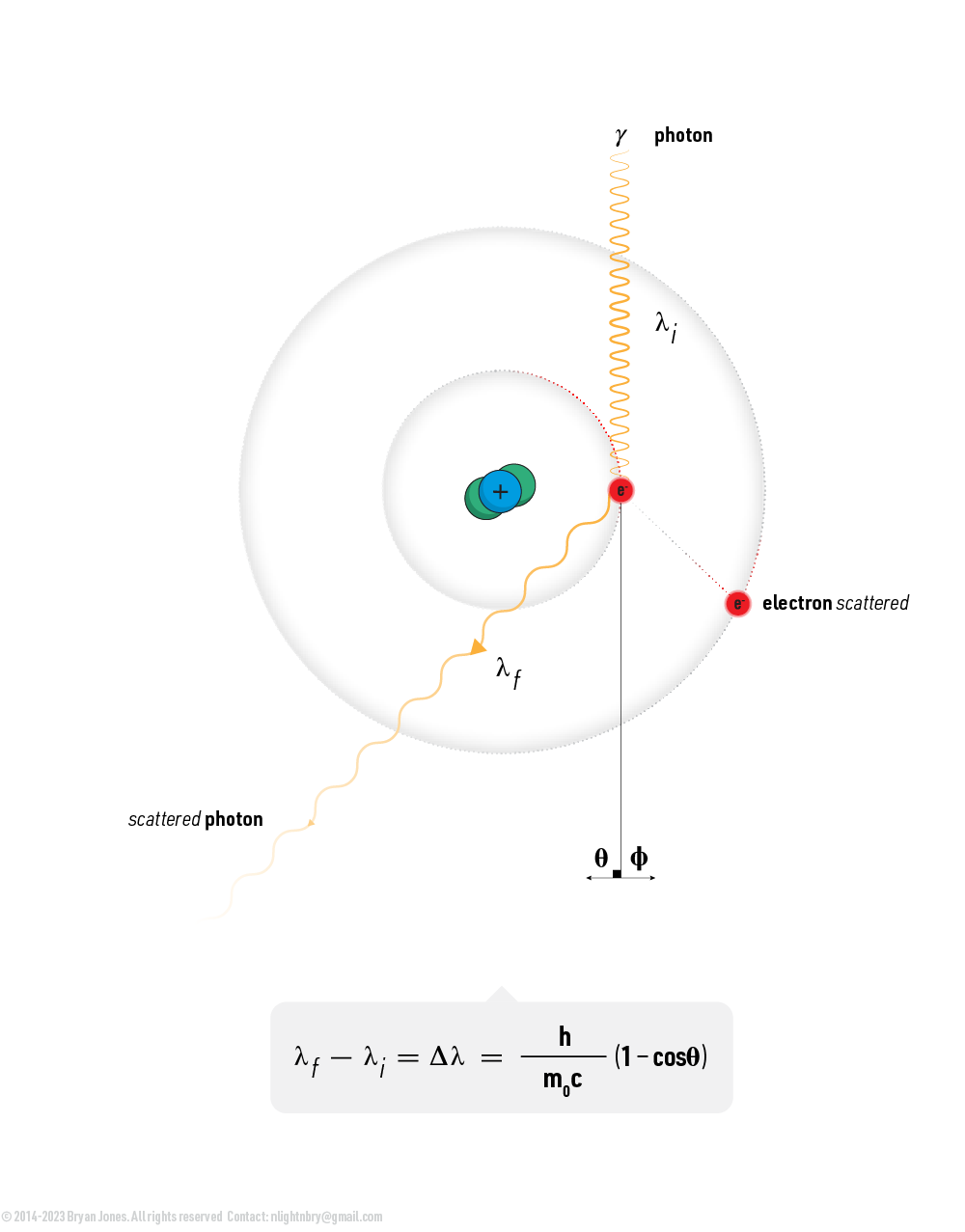
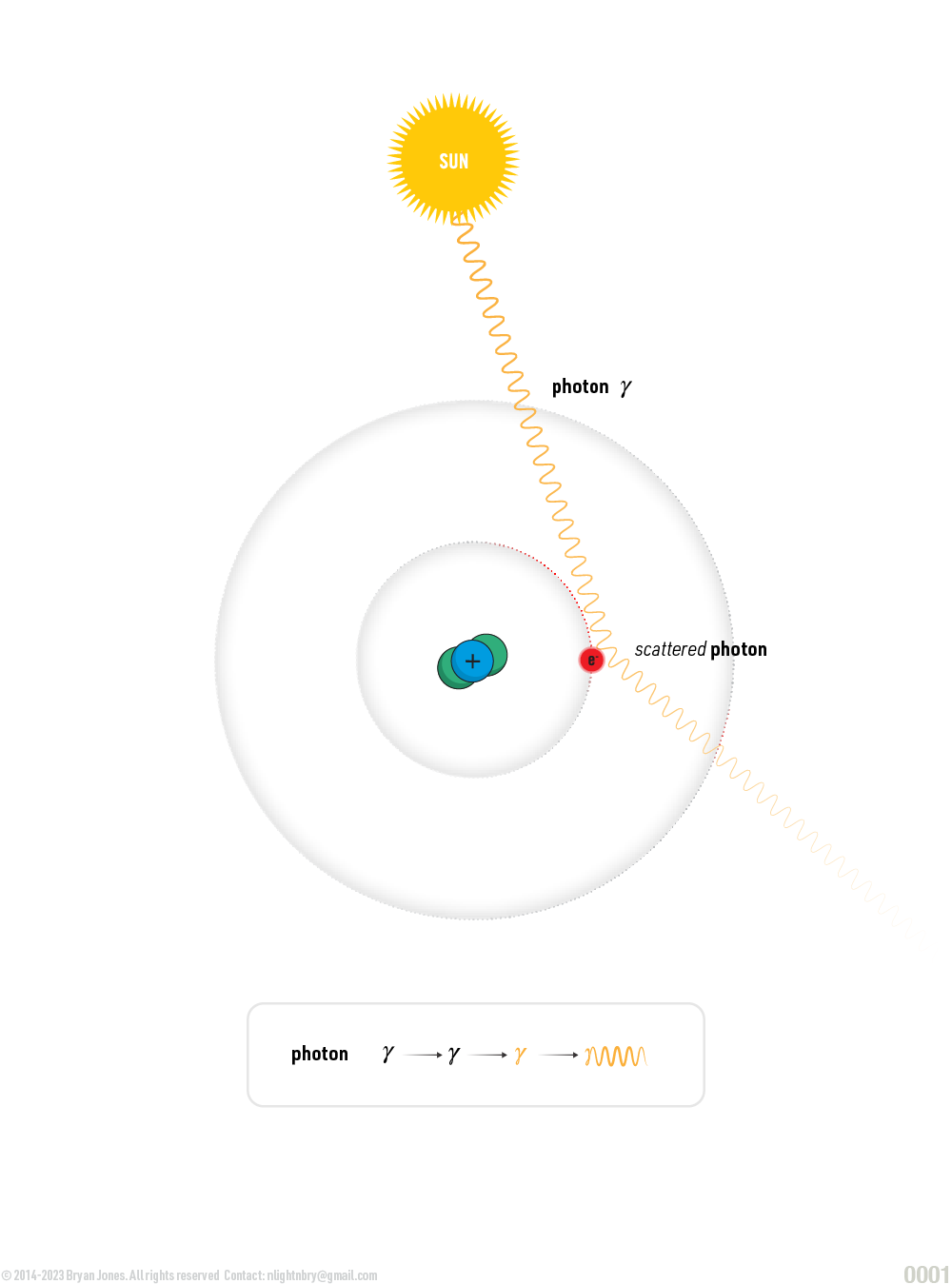
- Scattering: Photons can also scatter off of electrons in a material, changing the direction and energy of the photon. This can be elastic, meaning the photon's energy and wavelength remain the same, or inelastic, meaning the photon's energy and wavelength change.
- Photoelectric effect: When photons interact with metals, they can cause electrons to be emitted from the surface of the metal. This process is known as the photoelectric effect, and it occurs when the energy of the photon is greater than the work function of the metal.
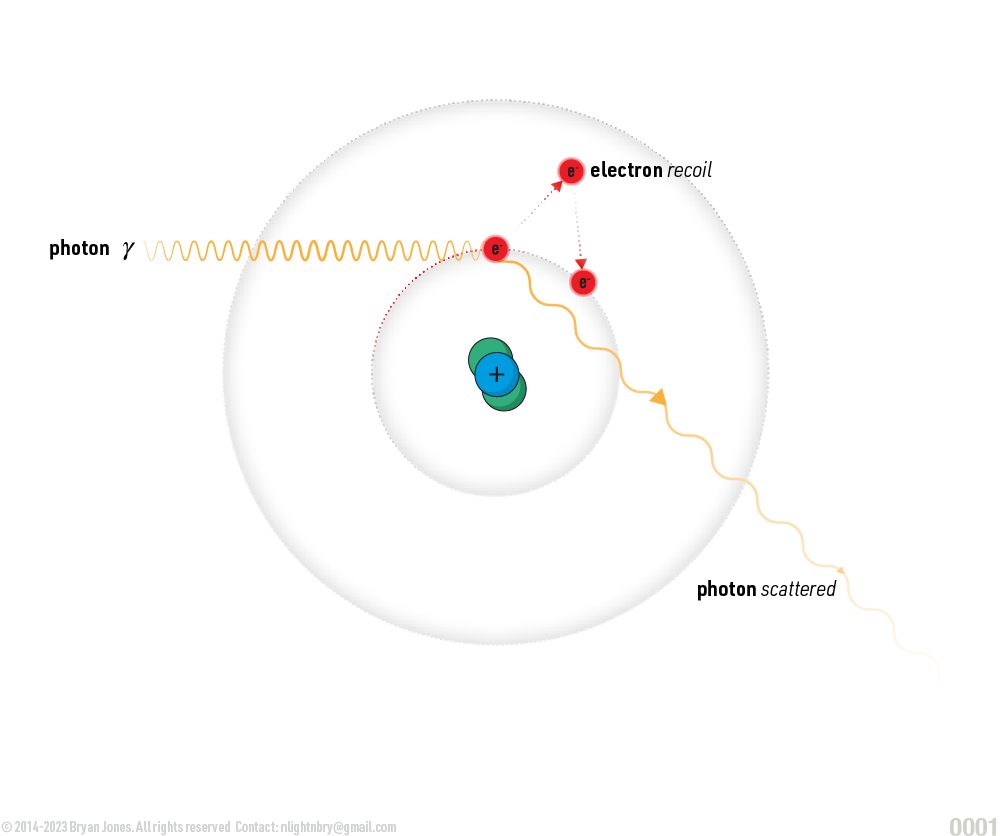
- Compton scattering: In Compton scattering, a photon interacts with a free electron, transferring some of its energy to the electron and causing it to recoil. The photon's energy and wavelength change as a result of the interaction.
Overall, the interactions between electrons and photons depend on the energy of the photons and the properties of the material that the electrons are in. These interactions are fundamental to many processes in physics, chemistry, and biology, including the absorption of light by plants for photosynthesis, the operation of solar cells, and the production of X-rays in medical imaging.
Emission: Conversely, an excited electron can release energy in the form of a photon, returning to a lower energy state. This process is known as emission or spontaneous emission.
- Electron moving around nucleus looses energy and drops to Shell 1
- Electron emits a photon which travels outward from nucleus
- Electron continues to orbit nucleus
Emission
Scattering: Photons can also scatter off of electrons in a material, changing the direction and energy of the photon. This can be elastic, meaning the photon's energy and wavelength remain the same, or inelastic, meaning the photon's energy and wavelength change. The shorter the wavelength of light, the higher its energy.
As light bounces off an object and hits your retina know that it started off in the center of a star, bounced its way through our atmosphere, collided with a leaf and then bounced in such a way that the photon hitting your retina has a wavelength of 520-565 nm which is green.
When a photon's velocity or more accurately its energy changes so does its color. If a photon traveling at ~0.93-1.10 eV which is violet light (380-435nm) collides with an electron it can lose energy in which violet light becomes blue, green, or even red.
Scattering
Absorption: When a photon interacts with an atom or molecule, it can be absorbed, transferring its energy to the electrons in the material. This can cause an electron to move to a higher energy level, or it can cause an electron to be ejected from the material altogether (photoelectric effect).
Photoelectric effect: When photons interact with metals, they can cause electrons to be emitted from the surface of the metal. This process is known as the photoelectric effect, and it occurs when the energy of the photon is greater than the work function of the metal.
Compton scattering: In Compton scattering, a photon interacts with a free electron, transferring some of its energy to the electron and causing it to recoil. The photon's energy and wavelength change as a result of the interaction.
Electron Shells
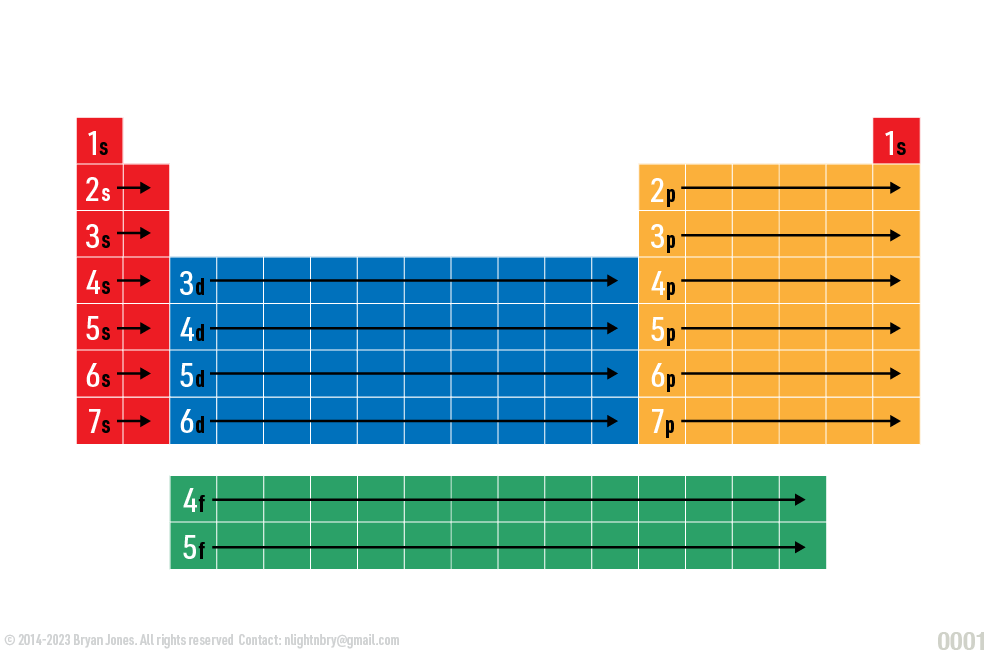
Electron orbitals are regions of space around an atom where electrons are likely to be found. There are four types of orbitals: s, p, d, and f. Each type of orbital has a different shape and can hold a different number of electrons.
Electron configuration is the arrangement of electrons in an atom. It is written in a standardized notation that shows the number of electrons in each energy level and subshell. The energy levels are labeled with the principal quantum number (n), and the subshells are labeled with the angular momentum quantum number (l). For example, the electron configuration of carbon (atomic number 6) is 1s22s2p2. This means that carbon has 2 electrons in the 1s subshell, 2 electrons in the 2s subshell, and 2 electrons in the 2p subshell.
Electron Configuration
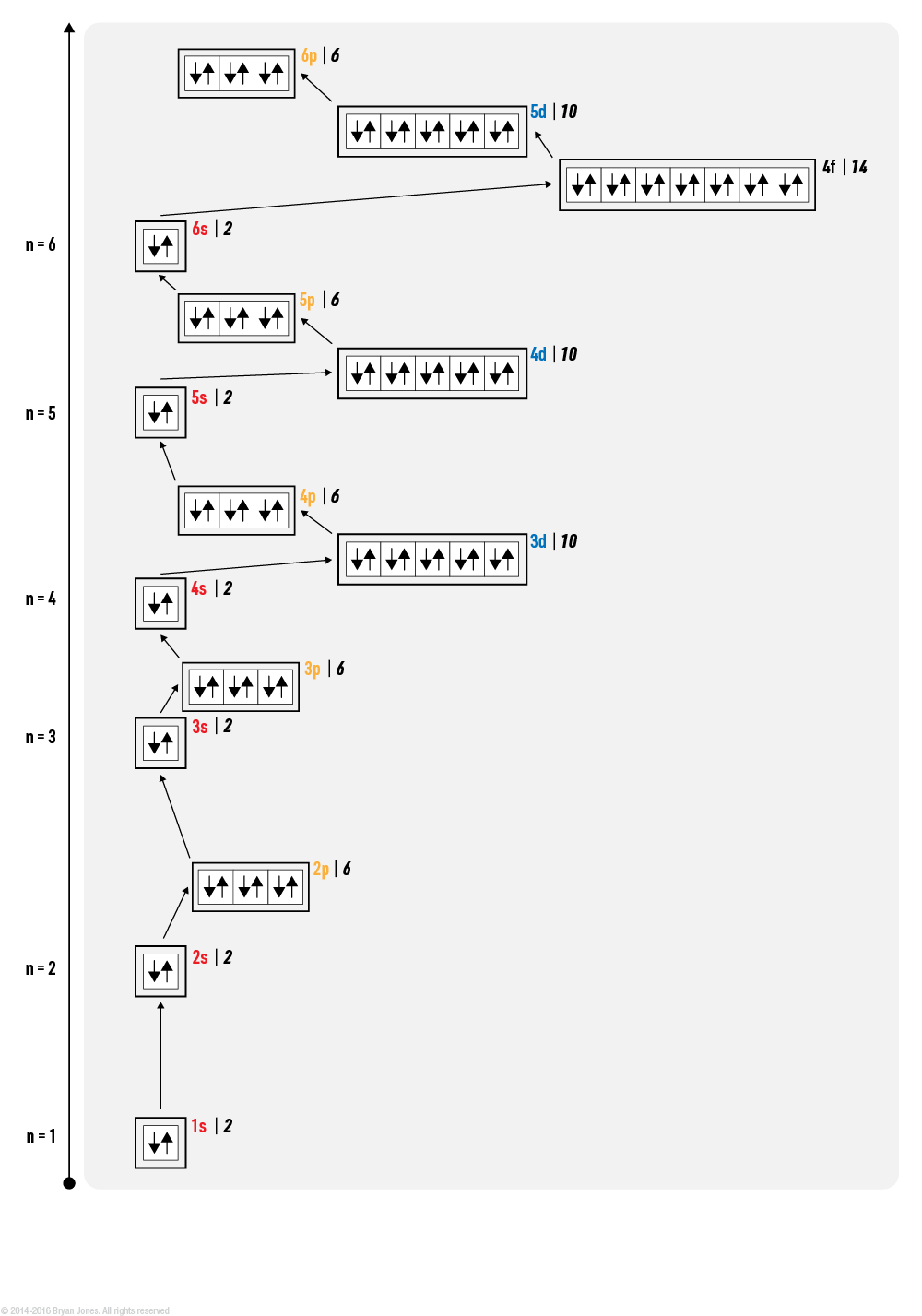
The order in which electrons fill the subshells is determined by the Aufbau principle, which states that electrons fill the lowest energy subshells first. The s subshell is the lowest energy subshell, followed by the p subshell, the d subshell, and the f subshell.
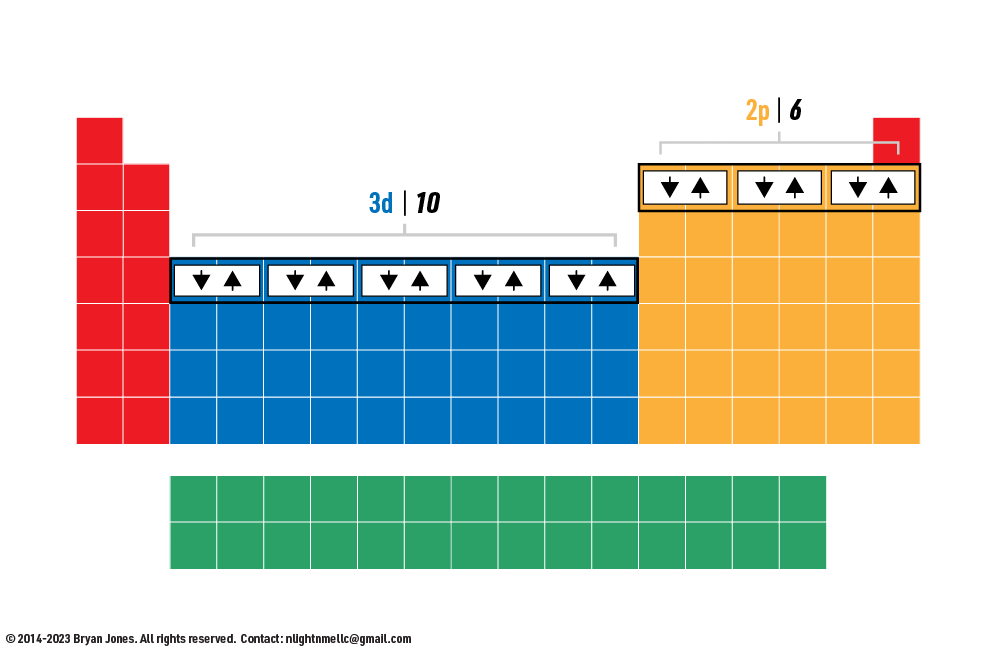
Electron configuration is important because it determines the chemical properties of an atom. The number of valence electrons (electrons in the outermost shell) determines how an atom will react with other atoms. For example, carbon has 4 valence electrons, which makes it a very reactive element. It can form bonds with other atoms to satisfy its need for 8 valence electrons.
Octet rule continued
Titanium Oxide

Energy values provided are approximate values for a hydrogen-like atom and are given in electron volts (eV). The table includes the primary orbitals for each energy level, but it does not cover all the possible sub-orbitals.
Electron Volts
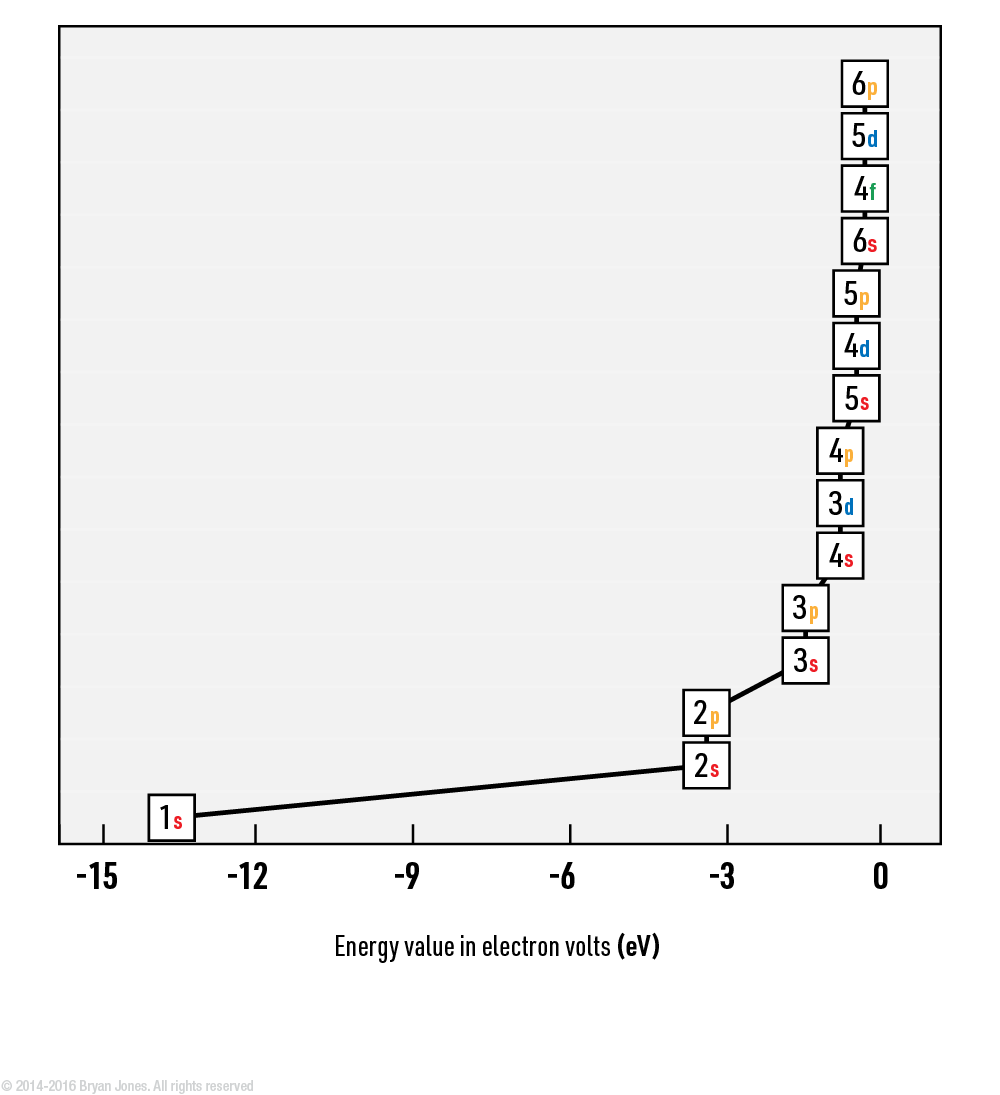
Energy values provided are approximate values for a hydrogen-like atom and are given in electron volts (eV). The table includes the primary orbitals for each energy level, but it does not cover all the possible sub-orbitals.
| Energy Level (n) | Orbital Designation | Energy Value (in electron volts, eV) |
|---|---|---|
| 1 | 1s | -13.6 |
| 2 | 2s | -3.4 |
| 2p | -3.4 | |
| 3 | 3s | -1.51 |
| 3p | -1.51 | |
| 4 | 4s | -0.85 |
| 3d | -0.85 | |
| 4p | -0.85 | |
| 5 | 5s | -0.54 |
| 4d | -0.54 | |
| 5p | -0.54 | |
| 6 | 6s | -0.38 |
| 4f | -0.38 | |
| 5d | -0.38 | |
| 6p | -0.38 |
- Is -13.6 eV more than -0.38 eV? As in does is take more Energy to fill 1s than 6p?
- No, the energy value in electron volts (eV) represents the energy required to remove an electron from an atom or the energy gained when an electron is added to an atom. In the case of hydrogen-like atoms, the energy levels become more negative (i.e., lower energy) as you move closer to the nucleus.
In this context, an energy level of -13.6 eV corresponds to the ground state energy level of the hydrogen atom, which is the most stable state. As you move towards higher energy levels, such as the 6p orbital with an energy value of -0.38 eV, the energy decreases, indicating a higher energy state.
Therefore, it takes less energy to fill the 6p orbital than it does to fill the 1s orbital. The 1s orbital is closer to the nucleus and has a more negative energy value, indicating a lower energy state and greater stability.
In this context, an energy level of -13.6 eV corresponds to the ground state energy level of the hydrogen atom, which is the most stable state. As you move towards higher energy levels, such as the 6p orbital with an energy value of -0.38 eV, the energy decreases, indicating a higher energy state.
Therefore, it takes less energy to fill the 6p orbital than it does to fill the 1s orbital. The 1s orbital is closer to the nucleus and has a more negative energy value, indicating a lower energy state and greater stability.
| Energy Level (n) | Orbital Designation | Maximum Number of Electrons |
|---|---|---|
| 1 | 1s | 2 |
| 2 | 2s | 2 |
| 2p | 6 | |
| 3 | 3s | 2 |
| 3p | 6 | |
| 4 | 4s | 2 |
| 3d | 10 | |
| 4p | 6 | |
| 5 | 5s | 2 |
| 4d | 10 | |
| 5p | 6 | |
| 6 | 6s | 2 |
| 4f | 14 | |
| 5d | 10 | |
| 6p | 6 | |
| 7 | 5f | 14 |
| 6d | 10 | |
| 7s | 2 | |
| 8 | 6f | 14 |
| 7p | 6 |
| Principal Quantum Number (n) | Orbital Type (l) | Subshell | Number of Orbitals | Maximum Number of Electrons |
|---|---|---|---|---|
| 1 | s | 1s | 1 | 2 |
| 2 | s | 2s | 1 | 2 |
| 2 | p | 2p | 3 | 6 |
| 3 | s | 3s | 1 | 2 |
| 3 | p | 3p | 3 | 6 |
| 3 | d | 3d | 5 | 10 |
| 4 | s | 4s | 1 | 2 |
| 4 | p | 4p | 3 | 6 |
| 4 | d | 4d | 5 | 10 |
| 4 | f | 4f | 7 | 14 |
| 5 | s | 5s | 1 | 2 |
| 5 | p | 5p | 3 | 6 |
| 5 | d | 5d | 5 | 10 |
| 5 | f | 5f | 7 | 14 |
| 6 | s | 6s | 1 | 2 |
| 6 | p | 6p | 3 | 6 |
| 6 | d | 6d | 5 | 10 |
| 6 | f | 6f | 7 | 14 |
| 6 | g | 6g | 9 | 18 |
| 7 | s | 7s | 1 | 2 |
| 7 | p | 7p | 3 | 6 |
| 7 | d | 7d | 5 | 10 |
| 7 | f | 7f | 7 | 14 |
| 7 | g | 7g | 9 | 18 |
| 8 | s | 8s | 1 | 2 |
| 8 | p | 8p | 3 | 6 |
| 8 | d | 8d | 5 | 10 |
| 8 | f | 8f | 7 | 14 |
| 8 | g | 8g | 9 | 18 |
- Is -13.6 eV more than -0.38 eV? As in does is take more Energy to fill 1s than 6p?
- No, the energy value in electron volts (eV) represents the energy required to remove an electron from an atom or the energy gained when an electron is added to an atom. In the case of hydrogen-like atoms, the energy levels become more negative (i.e., lower energy) as you move closer to the nucleus.
In this context, an energy level of -13.6 eV corresponds to the ground state energy level of the hydrogen atom, which is the most stable state. As you move towards higher energy levels, such as the 6p orbital with an energy value of -0.38 eV, the energy decreases, indicating a higher energy state.
Therefore, it takes less energy to fill the 6p orbital than it does to fill the 1s orbital. The 1s orbital is closer to the nucleus and has a more negative energy value, indicating a lower energy state and greater stability.
In this context, an energy level of -13.6 eV corresponds to the ground state energy level of the hydrogen atom, which is the most stable state. As you move towards higher energy levels, such as the 6p orbital with an energy value of -0.38 eV, the energy decreases, indicating a higher energy state.
Therefore, it takes less energy to fill the 6p orbital than it does to fill the 1s orbital. The 1s orbital is closer to the nucleus and has a more negative energy value, indicating a lower energy state and greater stability.
| Energy Level (n) | Orbital Designation | Maximum Number of Electrons |
|---|---|---|
| 1 | 1s | 2 |
| 2 | 2s | 2 |
| 2p | 6 | |
| 3 | 3s | 2 |
| 3p | 6 | |
| 4 | 4s | 2 |
| 3d | 10 | |
| 4p | 6 | |
| 5 | 5s | 2 |
| 4d | 10 | |
| 5p | 6 | |
| 6 | 6s | 2 |
| 4f | 14 | |
| 5d | 10 | |
| 6p | 6 | |
| 7 | 5f | 14 |
| 6d | 10 | |
| 7s | 2 | |
| 8 | 6f | 14 |
| 7p | 6 |
| Principal Quantum Number (n) | Orbital Type (l) | Subshell | Number of Orbitals | Maximum Number of Electrons |
|---|---|---|---|---|
| 1 | s | 1s | 1 | 2 |
| 2 | s | 2s | 1 | 2 |
| 2 | p | 2p | 3 | 6 |
| 3 | s | 3s | 1 | 2 |
| 3 | p | 3p | 3 | 6 |
| 3 | d | 3d | 5 | 10 |
| 4 | s | 4s | 1 | 2 |
| 4 | p | 4p | 3 | 6 |
| 4 | d | 4d | 5 | 10 |
| 4 | f | 4f | 7 | 14 |
| 5 | s | 5s | 1 | 2 |
| 5 | p | 5p | 3 | 6 |
| 5 | d | 5d | 5 | 10 |
| 5 | f | 5f | 7 | 14 |
| 6 | s | 6s | 1 | 2 |
| 6 | p | 6p | 3 | 6 |
| 6 | d | 6d | 5 | 10 |
| 6 | f | 6f | 7 | 14 |
| 6 | g | 6g | 9 | 18 |
| 7 | s | 7s | 1 | 2 |
| 7 | p | 7p | 3 | 6 |
| 7 | d | 7d | 5 | 10 |
| 7 | f | 7f | 7 | 14 |
| 7 | g | 7g | 9 | 18 |
| 8 | s | 8s | 1 | 2 |
| 8 | p | 8p | 3 | 6 |
| 8 | d | 8d | 5 | 10 |
| 8 | f | 8f | 7 | 14 |
| 8 | g | 8g | 9 | 18 |
Atomic Species
Remember, these terms are specific to the field of atomic and molecular species and may not encompass all possible terms related to this subject.
| Category | Definition |
|---|---|
| Isotopes | Isotopes are different forms of an element that have the same number of protons but different numbers of neutrons in their atomic nuclei. This difference in neutron numbers results in isotopes having different atomic masses. For example, carbon-12 and carbon-14 are isotopes of carbon. |
| Isobars | Isobars are atoms of different elements that have the same mass number but different atomic numbers. In other words, isobars have the same total number of nucleons (protons + neutrons) but different numbers of protons. For example, carbon-14 and nitrogen-14 are isobars because they both have a mass number of 14, but carbon-14 has 6 protons and nitrogen-14 has 7 protons. |
| Isodiaphers | The term "isodiaphers" is not commonly used in the context of nuclear physics or chemistry. It does not have a widely recognized definition or specific meaning. |
| Isoelectronic | Isoelectronic refers to different atoms, ions, or molecules that have the same number of electrons. Although these species may have different numbers of protons and neutrons, their electron configurations are identical. This similarity in electron configuration often leads to similar chemical properties among isoelectronic species. |
| Isotones | Isotones are atoms or nuclei that have the same number of neutrons but different numbers of protons. In other words, isotones have the same neutron number but different atomic numbers. For example, carbon-13 and nitrogen-14 are isotones because they both have 7 neutrons, but carbon-13 has 6 protons and nitrogen-14 has 7 protons. |
| Isomers | Isomers are different chemical species that have the same molecular formula but different arrangements or spatial orientations. Isomers can have distinct chemical and physical properties despite having the same atoms and the same number of each atom. |
| Isosters | Isosters are molecules or ions that have the same total number of valence electrons and similar structural arrangements. Isosters can differ in the types of atoms present but exhibit similar electronic structures and often similar chemical properties. |
| Isomorphs | Isomorphs are crystalline substances that have similar crystal structures but may contain different chemical elements or compounds. Isomorphs can have comparable crystal lattices and similar physical properties, such as density and hardness. |
| Isostructural | Isostructural compounds or substances have the same or very similar crystal structures, despite having different chemical compositions. Isostructural compounds often exhibit similar arrangement of atoms in the crystal lattice, resulting in comparable physical and structural properties. |
Structural Magnitudes
Atoms are full of even smaller units, and those units have even smaller units. Simple units make larger units that can behave similarly, even at different scales. For example, the torus shape occurs at drastically different scales.
Torus Examples
| Example | Scale |
|---|---|
| Bacterium MOT complex | 100 nm | Vaterite toroid | 6 μm |
| Doughnuts you eat | 10 cm |
| Electric motor | 1 cm - 10 m |
| Möbius strip shaped toroid used in fission | 7-18 m |
| Torus shaped matter surrounding a blackhole | 100,000,000,000,000 m |
The concept of forces, fields, and attraction can be difficult to perceive at first, with continue practice and time it becomes clearer. It helps to know that the universe tends to maintain balance. Not only that, but the universe is similar a Möbius strip and Klein bottle (Delicata Squash) and many other considerations have been theorized.
It is important to mention that the universe generates many shapes, not just Euclidean shapes. Any conceivable shape is relative to the universe. The universe cannot be simplified down to a Klein bottle. Instead, it is likely a shape that merges every perceivable shape into one shape, and other shapes simultaneously, including shapes that are not technically referred to as shapes.
Fundamental Forces
- Gravity is the force that attracts all objects with mass towards each other. It is the weakest of the four fundamental forces, but it is also the most long-range.
- Electromagnetism is the force that binds electrons to protons in atoms, and it is also responsible for light and other forms of radiation. It is much stronger than gravity, but it has a much shorter range.
- The strong force is the force that binds quarks together to form protons and neutrons, and it is also responsible for holding the nucleus of an atom together. It is the strongest of the four fundamental forces, but it only has a very short range.
- The weak force is the force that is responsible for radioactive decay and other processes that involve the transformation of one type of subatomic particle into another. It is much weaker than the strong force and electromagnetism, but it has a slightly longer range.
| Force | Strength | Range |
|---|---|---|
| Gravity | Weakest | Longest |
| Electronmagnetism | Stronger | Shorter |
| Strong force | Strongest | Very short |
| Weak Force | Weaker | Slightly longer |
Contents
| Frequency | Order | Name 1 | Name 2 | Name 3 | Standing wave representation | Longitudinal wave representation |
|---|---|---|---|---|---|---|
| 1 × f = 440 Hz | n = 1 | 1st partial | fundamental tone | 1st harmonic | 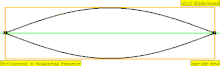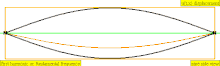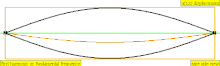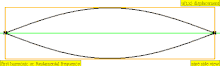
|

|
| 2 × f = 880 Hz | n = 2 | 2nd partial | 1st overtone | 2nd harmonic | 
|

|
| 3 × f = 1320 Hz | n = 3 | 3rd partial | 2nd overtone | 3rd harmonic | 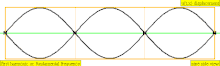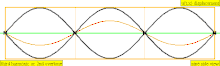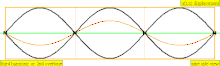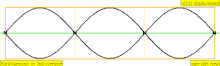
|

|
| 4 × f = 1760 Hz | n = 4 | 4th partial | 3rd overtone | 4th harmonic | 
|
|
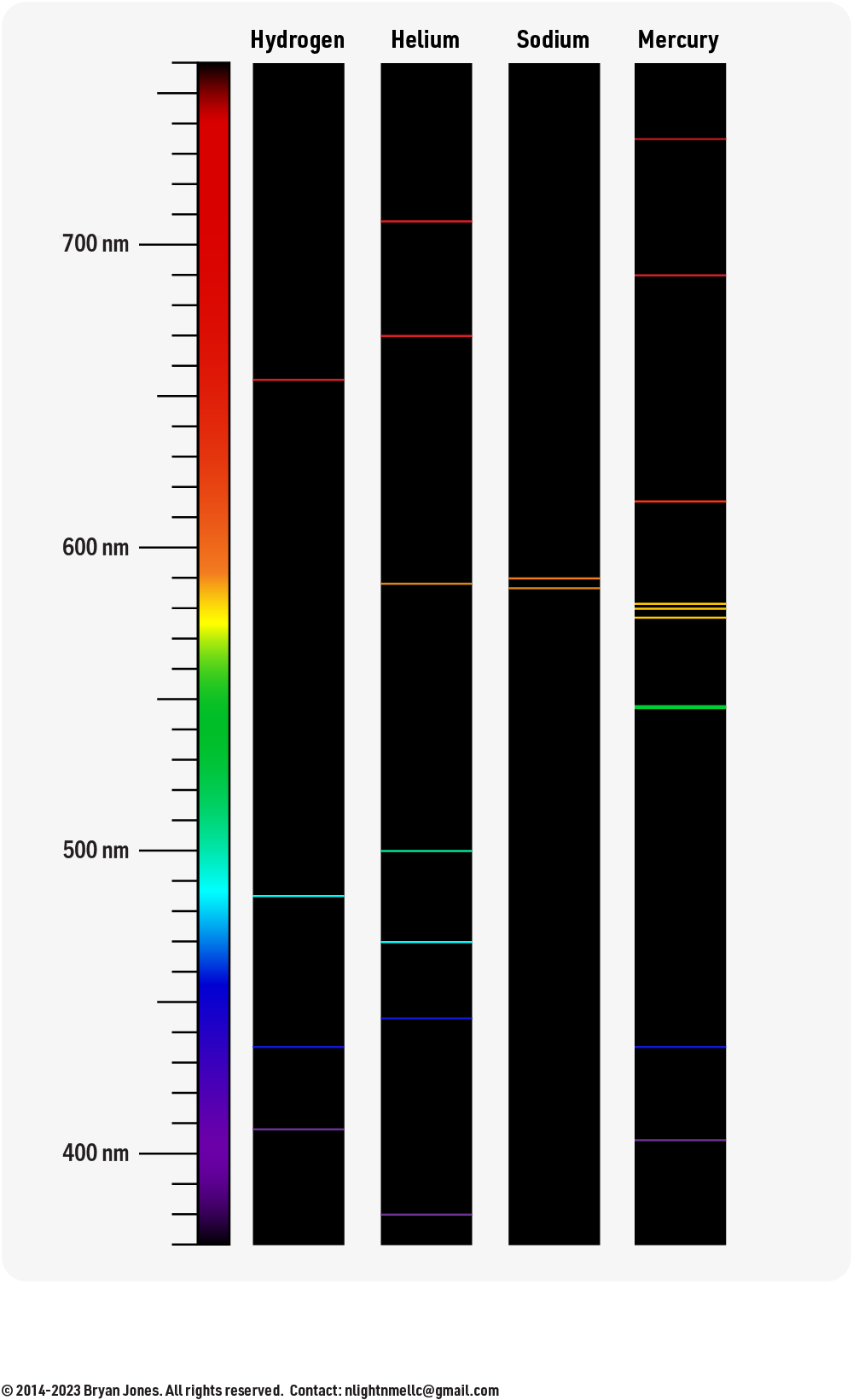
Spectroscopy
Emission Spectra